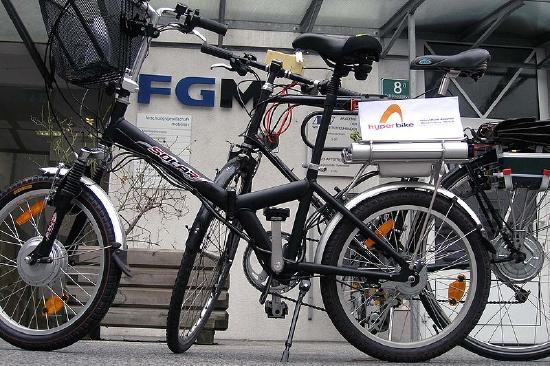Electric vehicles of all kinds are attracting increased interest everywhere, but electric bicycles are very popular in China (where there are over 120 million of them), the Netherlands, and India. They typically have a rechargeable battery pack and electric hub motor.
Electric hub motors on front or back wheels
A new electricity source combines a hydrogen fuel cell with a "sodium silicide" fuel cartridge, winner of a "Green Chemistry Challenge Award"[1]. The sodium silicyde reacts with water to make the hydrogen fuel [2][3][4]:
- 2 NaSi(s) + 5H2O(l) → Na2Si2O5(s) + 5H2(g) (1)
The composition of sodium silicide may depend on the method of synthesis. Silicides can be made by the reaction of active metals (like Mg) with sand, or by heating sodium with silicon. Dye et al [5] prepare sodium silicide by the reaction of sodium metal with silica gel, obtaining black powders of (hypothetically) Na4Si4 nanoparticles.
Equation (1) not only tells how many molecules of each kind are involved in a reaction, it also indicates the amount of each substance that is involved. Equation (1) says that 2 NaSi formula units can react with 5 H2O molecules to give 1 Na2Si2O5(s) formula unit and 5 H2 molecules. Here we're using the term "formula unit" to indicate that the substance may not be a molecule, but rather an ionic compound or ["network crystal"]. A "formula unit" gives the composition of the substance without specifying the type of bonding.
Equation (1) also says that 1 mol NaSi would react with 5 mol H2O yielding 1 mol Na2Si2O5(s) and 5 mol H2.
The balanced equation does more than this, though. It also tells us that 2 × 2 mol = 4 mol NaSi will react with 2 × 5 mol = 10 mol H2O, and that ½ × 2 mol = 1 mol NaSi requires only ½ × 5 = 2.5 mol H2O. In other words, the equation indicates that exactly 5 mol H2O must react for every 2 mol NaSi consumed. For the purpose of calculating how much H2O is required to react with a certain amount of NaSi therefore, the significant information contained in Eq. (1) is the ratio
\(\frac{\text{5 mol H}_{\text{2}}\text{O}}{\text{2 mol NaSi}}\) We shall call such a ratio derived from a balanced chemical equation a stoichiometric ratio and give it the symbol S. Thus, for Eq. (1), \(\text{S}\left( \frac{\text{H}_{\text{2}}\text{O}}{\text{NaSi}} \right)=\frac{\text{5 mol H}_{\text{2}}\text{O}}{\text{2 mol NaSi}}~~~~~ \text{(2)}\)
The word stoichiometric comes from the Greek words stoicheion, “element,“ and metron, “measure.“ Hence the stoichiometric ratio measures one element (or compound) against another.
EXAMPLE 1
Derive all possible stoichiometric ratios from Eq. (1)
Solution
Any ratio of amounts of substance given by coefficients in the equation may be used:
\(\text{S}\left( \frac{\text{NaSi}}{\text{H}_{\text{2}}\text{O}} \right) =\frac{\text{2 mol NaSi}}{\text{5 mol H}_{\text{2}}\text{O}}\) \(\text{S}\left( \frac{\text{H}_{\text{2}}\text{O}}{\text{Na}_{2}\text{Si}_{2}\text{O}_{5}} \right) =\frac{\text{5 mol H}_{\text{2}}\text{O}}{\text{1 mol Na}_{2}\text{Si}_{2}\text{O}_{2}}\)
\(\text{S}\left( \frac{\text{NaSi}}{\text{Na}_{2}\text{Si}_{2}\text{O}_{5}} \right) =\frac{\text{2 mol NaSi}}{\text{1 mol Na}_{2}\text{Si}_{2}\text{O}_{5}}\) \(\text{S}\left( \frac{\text{H}_{\text{2}}\text{O}}{\text{H}_{\text{2}}} \right)=\frac{\text{5 mol H}_{\text{2}}\text{O}}{\text{5 mol H}_{\text{2}}}\) \(\text{S}\left( \frac{\text{NaSi}}{\text{H}_{\text{2}}} \right) =\frac{\text{2 mol NaSi}}{\text{5 mol H}_{\text{2}}}\) \(\text{S}\left( \frac{\text{Na}_{2}\text{Si}_{2}\text{O}_{5}}{\text{H}_{\text{2}}} \right) =\frac{\text{1 mol Na}_{2}\text{Si}_{2}\text{O}_{5}}{\text{5 mol H}_{\text{2}}}\) There are six more stoichiometric ratios, each of which is the reciprocal of one of these. [Eq. (2) gives one of them.] When any chemical reaction occurs, the amounts of substances consumed or produced are related by the appropriate stoichiometric ratios. Using Eq. (1) as an example, this means that the ratio of the amount of H2O consumed to the amount of NaSi consumed must be the stoichiometric ratio S(H2O/NaSi): \(\frac{n_{\text{H}_{\text{2}}\text{O}\text{ consumed}}}{n_{\text{NaSi}\text{ consumed}}}=\text{S}\left( \frac{\text{H}_{\text{2}}\text{O}}{\text{NaSi}} \right) =\frac{\text{5 mol H}_{\text{2}}\text{O}}{\text{2 mol NaSi}}\) Similarly, the ratio of the amount of H2 produced to the amount of NaSi consumed must be
S(H2/NaSi):
\(\frac{n_{\text{H}_{\text{2}}\text{ produced}}}{n_{\text{NaSi}\text{ consumed}}} =\text{S}\left( \frac{\text{H}_{\text{2}}}{\text{NaSi}} \right) =\frac{\text{5 mol H}_{\text{2}}}{\text{2 mol NaSi}}\) In general we can say that \(\text{Stoichiometric ratio }\left( \frac{\text{X}}{\text{Y}} \right)=\frac{\text{amount of X consumed or produced}}{\text{amount of Y consumed or produced}}~~~~~\text{(3a)}\) or, in symbols, \(\text{S}\left( \frac{\text{X}}{\text{Y}} \right)=\frac{n_{\text{X consumed or produced}}}{n_{\text{Y consumed or produced}}}~~~~~\text{(3b)}\)
Note that in the word Eq. (3a) and the symbolic Eq. (3b), X and Y may represent any reactant or any product in the balanced chemical equation from which the stoichiometric ratio was derived. No matter how much of each reactant we have, the amounts of reactants consumed and the amounts of products produced will be in appropriate stoichiometric ratios.
EXAMPLE 2
Find the amount of hydrogen produced when 3.68 mol NaSi is consumed according to Eq. (1).
Solution
The amount of hydrogen produced must be in the stoichiometric ratio S(H2/NaSi) to the amount of ammonia consumed:
\(\text{S}\left( \frac{\text{H}_{\text{2}}}{\text{NaSi}} \right) =\frac{n_{\text{H}_{\text{2}}\text{ produced}}}{n_{\text{NaSi}\text{ consumed}}}\)
Multiplying both sides nNaSi consumed, by we have \(n_{\text{H}_{\text{2}}\text{ produced}} =n_{\text{NaSi}\text{ consumed}}\times \text{S}\left( \frac{\text{H}_{\text{2}}}{\text{NaSi}} \right) =\text{3}\text{.68 mol NaSi}\times \frac{\text{5 mol H}_{\text{2}}}{\text{2 mol NaSi}}=\text{9}\text{.20 mol H}_{\text{2}}\) This is a typical illustration of the use of a stoichiometric ratio as a conversion factor. Example 2 is analogous to Examples 1 and 2 from Conversion Factors and Functions, where density was employed as a conversion factor between mass and volume. Example 2 is also analogous to Examples 2.4 and 2.6, in which the Avogadro constant and molar mass were used as conversion factors. As in these previous cases, there is no need to memorize or do algebraic manipulations with Eq. (3) when using the stoichiometric ratio. Simply remember that the coefficients in a balanced chemical equation give stoichiometric ratios, and that the proper choice results in cancellation of units. In road-map form \(\text{amount of X consumed or produced}\overset{\begin{smallmatrix} \text{stoichiometric} \\ \text{ ratio X/Y} \end{smallmatrix}}{\longleftrightarrow}\text{amount of Y consumed or produced}\) or symbolically. \(n_{\text{X consumed or produced}}\text{ }\overset{S\text{(X/Y)}}{\longleftrightarrow}\text{ }n_{\text{Y consumed or produced}}\)
When using stoichiometric ratios, be sure you always indicate moles of what. You can only cancel moles of the same substance. In other words, 1 mol NaSi cancels 1 mol NaSi but does not cancel 1 mol H2.
The next example shows that stoichiometric ratios are also useful in problems involving the mass of a reactant or product.
EXAMPLE 3
Suppose it is reasonable to carry about 5 pounds (2268 g) of water on a bicycle for "fuel". Calculate the mass of NaSi that needs to be supplied by a cartridge on the bicycle to completely react with the water.
The problem asks that we calculate the mass of NaSi consumed from the mass of H2O consumed. As we learned in Example 2 of The Molar Mass, the molar mass can be used to convert from the mass of water to the amount of water. We can then use the appropriate stoichiometric ratio to calculate the amount of NaSi that will react, and finally, use the molar mass to calculate the mass of NaSi.
We require the stoichiometric ratio
\(\text{S}\left( \frac{\text{NaSi}}{\text{H}_{\text{2}}} \right) =\frac{\text{2 mol NaSi}}{\text{5 mol H}_{\text{2}}}\)
The amount of H2 present is
\(\text{n(mol)}~=~\frac{\text{m(g)}}{\text{M(g/mol)}}\)
= 2268 g/18.015 g/mol = 125.9 mol H2O
The amount of NaSi required is then
\(n_{\text{NaSi consumed}} ~=~n_{\text{H}_{2}\text{O consumed}}~~\times~~\text{ conversion factor}\)
\(=\text{125.9 mol H}_{2}\text{O}~~\times~~\frac{\text{2 mol NaSi}}{\text{5 mol H}_{\text{2}}\text{O}} =\text{50.36 mol NaSi}\)
The mass of NaSi is \(\text{m}_{\text{NaSi}}=\text{50}\text{.36 mol NaSi}\times \frac{\text{51}\text{.08 g NaSi}}{\text{1 mol NaSI}}=\text{2572 g NaSi}\)
This is a reasonably sized cartridge (about 5.67 lb).
With practice this kind of problem can be solved in one step by concentrating on the units. The appropriate stoichiometric ratio will convert moles of H2O to moles of NaSi and the molar mass will convert moles of NaSi to grams of NaSi. A schematic road map for the one-step calculation can be written as
\(n_{\text{H}_{2}\text{O}}~~\xrightarrow{S\text{(NaSi}\text{/H}_{\text{2}}\text{O)}}~~n_{\text{NaSi}}~~\xrightarrow{M_{\text{NaSi}}}~~m_{\text{NaSi}}\) Thus \(\text{m}_{\text{NaSi}}=\text{125.9 mol H}_{\text{2}}\text{O}\times~~\frac{\text{2 mol NaSi}}{\text{5 mol H}_{\text{2}}\text{O}}~~\times~~\frac{\text{51.06 g}}{\text{1 mol NaSi}}=\text{2572 g NaSi}\) These calculations can be organized as a table, with entries below the respective reactants and products in the chemical equation. You may verify the additional calculations that have been done to show the masses of hydrogen product that would be expected. We'll fill in the remaining spots below.
| |
2 NaSi |
+ 5 H2O → |
1 Na2Si3O5 |
+ 5 H2 |
| m (g) |
2572 |
2268 |
|
253.8 |
| M (g/mol) |
51.08 |
18.015 |
182.15 |
2.016 |
| n (mol) |
50.36 |
125.9 |
|
125.9 |
EXAMPLE 4
Suppose the 2572 g cannister of NaSi in Example 3 is completely depleted, which means completely converted to Na2Si2O5. What mass of the product results?
The problem gives the mass of NaSi and asks for the mass of Na2Si2O5 that would result from it's complete reaction with water. Thinking the problem through before trying to solve it, we realize that the molar mass of NaSi could be used to calculate the amount of NaSi consumed. Then we need a stoichiometric ratio to get the amount of Na2Si2O5 produced. Finally, the molar mass of Na2Si2O5 permits calculation of the mass of Na2Si2O5. Symbolically
\(m_{\text{NaSi}}~~\) \(\xrightarrow{M_{\text{NaSi}}}~~\) nNaSi \(~~\xrightarrow{S\text{(Na}_{2}\text{Si}_{2}\text{O}_{5}\text{/NaSi)}}\) \(~~n_{\text{Na}_{\text{2}}\text{Si}_{2}\text{O}_{5}}\) \(~~\xrightarrow{M_{\text{Na}_{2}\text{Si}_{2}\text{O}_{5}}}\) \(~~m_{\text{Na}_{2}\text{Si}_{2}\text{O}_{5}}\)
\(m_{\text{Na}_{2}\text{Si}_{2}\text{O}_{5}}\) \(=\text{2572 g }~~\times~~\frac{\text{1 mol NaSi}}{\text{58.08 g}}\) \(~~\times~~\frac{\text{1 mol Na}_{2}\text{Si}_{2}\text{O}_{5}}{\text{2 mol NaSi}}\) \(~~\times~~\frac{\text{182.14 g}}{\text{1 mol Na}_{2}\text{Si}_{2}\text{O}_{5}} =\text{4033 g }\)
Now we can complete the table above by adding the amount of Na2Si2O5 (25.18 mol, half the amount of NaSi) and its mass, 4033 g (or about 8.9 lb). Will the bike have gained weight, since the cartridge went from 2572 g of NaSi to 4033 g of Na2Si2O5?