23.8: Chemistry of Selected Transition Metals
- Page ID
- 91365
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- To use periodic trends to understand the chemistry of the transition metals.
As we shall see, the two heaviest members of each group usually exhibit substantial similarities in chemical behavior and are quite different from the lightest member.
Group 3 (Sc, Y, La, and Ac)
As shown in Table \(\PageIndex{1}\), the observed trends in the properties of the group 3 elements are similar to those of groups 1 and 2. Due to their ns2(n − 1)d1 valence electron configurations, the chemistry of all four elements is dominated by the +3 oxidation state formed by losing all three valence electrons. As expected based on periodic trends, these elements are highly electropositive metals and powerful reductants, with La (and Ac) being the most reactive. In keeping with their highly electropositive character, the group 3 metals react with water to produce the metal hydroxide and hydrogen gas:
\[2M_{(s)} + 6H_2O_{(l)} \rightarrow 2M(OH)_{3(s)} + 3H_{2(g)}\label{Eq1}\]
The chemistry of the group 3 metals is almost exclusively that of the M3+ ion; the elements are powerful reductants.
Moreover, all dissolve readily in aqueous acid to produce hydrogen gas and a solution of the hydrated metal ion: M3+(aq).
| Group | Element | Z | Valence Electron Configuration | Electronegativity | Metallic Radius (pm) | Melting Point (°C) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| 3 | Sc | 21 | 4s23d1 | 1.36 | 162 | 1541 | 2.99 |
| Y | 39 | 5s24d1 | 1.22 | 180 | 1522 | 4.47 | |
| La | 57 | 6s25d1 | 1.10 | 183 | 918 | 6.15 | |
| Ac | 89 | 7s26d1 | 1.10 | 188 | 1051 | 10.07 | |
| 4 | Ti | 22 | 4s23d2 | 1.54 | 147 | 1668 | 4.51 |
| Zr | 40 | 5s24d2 | 1.33 | 160 | 1855 | 6.52 | |
| Hf | 72 | 6s25d24f14 | 1.30 | 159 | 2233 | 13.31 | |
| 5 | V | 23 | 4s23d3 | 1.63 | 134 | 1910 | 6.00 |
| Nb | 41 | 5s24d3 | 1.60 | 146 | 2477 | 8.57 | |
| Ta | 73 | 6s25d34f14 | 1.50 | 146 | 3017 | 16.65 |
The group 3 metals react with nonmetals to form compounds that are primarily ionic in character. For example, reacting group 3 metals with the halogens produces the corresponding trihalides: MX3. The trifluorides are insoluble in water because of their high lattice energies, but the other trihalides are very soluble in water and behave like typical ionic metal halides. All group 3 elements react with air to form an oxide coating, and all burn in oxygen to form the so-called sesquioxides (M2O3), which react with H2O or CO2 to form the corresponding hydroxides or carbonates, respectively. Commercial uses of the group 3 metals are limited, but “mischmetal,” a mixture of lanthanides containing about 40% La, is used as an additive to improve the properties of steel and make flints for cigarette lighters.
Group 4 (Ti, Zr, and Hf)
Because the elements of group 4 have a high affinity for oxygen, all three metals occur naturally as oxide ores that contain the metal in the +4 oxidation state resulting from losing all four ns2(n − 1)d2 valence electrons. They are isolated by initial conversion to the tetrachlorides, as shown for Ti:
\[2FeTiO_{3(s)} + 6C_{(s)} + 7Cl_{2(g)} \rightarrow 2TiCl_{4(g)} + 2FeCl_{3(g)} + 6CO_{(g)}\label{Eq2}\]
followed by reduction of the tetrachlorides with an active metal such as Mg.
The chemistry of the group 4 metals is dominated by the +4 oxidation state. Only Ti has an extensive chemistry in lower oxidation states.
In contrast to the elements of group 3, the group 4 elements have important applications. Titanium (melting point = 1668°C) is often used as a replacement for aluminum (melting point = 660°C) in applications that require high temperatures or corrosion resistance. For example, friction with the air heats the skin of supersonic aircraft operating above Mach 2.2 to temperatures near the melting point of aluminum; consequently, titanium is used instead of aluminum in many aerospace applications. The corrosion resistance of titanium is increasingly exploited in architectural applications, as shown in the chapter-opening photo. Metallic zirconium is used in UO2-containing fuel rods in nuclear reactors, while hafnium is used in the control rods that modulate the output of high-power nuclear reactors, such as those in nuclear submarines.
Consistent with the periodic trends shown in Figure 23.2, the group 4 metals become denser, higher melting, and more electropositive down the column (Table \(\PageIndex{1}\)). Unexpectedly, however, the atomic radius of Hf is slightly smaller than that of Zr due to the lanthanide contraction. Because of their ns2(n − 1)d2 valence electron configurations, the +4 oxidation state is by far the most important for all three metals. Only titanium exhibits a significant chemistry in the +2 and +3 oxidation states, although compounds of Ti2+ are usually powerful reductants. In fact, the Ti2+(aq) ion is such a strong reductant that it rapidly reduces water to form hydrogen gas.
Reaction of the group 4 metals with excess halogen forms the corresponding tetrahalides (MX4), although titanium, the lightest element in the group, also forms dihalides and trihalides (X is not F). The covalent character of the titanium halides increases as the oxidation state of the metal increases because of increasing polarization of the anions by the cation as its charge-to-radius ratio increases. Thus TiCl2 is an ionic salt, whereas TiCl4 is a volatile liquid that contains tetrahedral molecules. All three metals react with excess oxygen or the heavier chalcogens (Y) to form the corresponding dioxides (MO2) and dichalcogenides (MY2). Industrially, TiO2, which is used as a white pigment in paints, is prepared by reacting TiCl4 with oxygen at high temperatures:
\[TiCl_{4(g)} + O_{2(g)} \rightarrow TiO_{2(s)} + 2Cl_{2(g)}\label{Eq3}\]
The group 4 dichalcogenides have unusual layered structures with no M–Y bonds holding adjacent sheets together, which makes them similar in some ways to graphite (Figure \(\PageIndex{1}\)). The group 4 metals also react with hydrogen, nitrogen, carbon, and boron to form hydrides (such as TiH2), nitrides (such as TiN), carbides (such as TiC), and borides (such as TiB2), all of which are hard, high-melting solids. Many of these binary compounds are nonstoichiometric and exhibit metallic conductivity.

Group 5 (V, Nb, and Ta)
Like the group 4 elements, all group 5 metals are normally found in nature as oxide ores that contain the metals in their highest oxidation state (+5). Because of the lanthanide contraction, the chemistry of Nb and Ta is so similar that these elements are usually found in the same ores.
Three-fourths of the vanadium produced annually is used in the production of steel alloys for springs and high-speed cutting tools. Adding a small amount of vanadium to steel results in the formation of small grains of V4C3, which greatly increase the strength and resilience of the metal, especially at high temperatures. The other major use of vanadium is as V2O5, an important catalyst for the industrial conversion of SO2 to SO3 in the contact process for the production of sulfuric acid. In contrast, Nb and Ta have only limited applications, and they are therefore produced in relatively small amounts. Although niobium is used as an additive in certain stainless steels, its primary application is in superconducting wires such as Nb3Zr and Nb3Ge, which are used in superconducting magnets for the magnetic resonance imaging of soft tissues. Because tantalum is highly resistant to corrosion, it is used as a liner for chemical reactors, in missile parts, and as a biologically compatible material in screws and pins for repairing fractured bones.
The chemistry of the two heaviest group 5 metals (Nb and Ta) is dominated by the +5 oxidation state. The chemistry of the lightest element (V) is dominated by lower oxidation states, especially +4.
As indicated in Table \(\PageIndex{1}\), the trends in properties of the group 5 metals are similar to those of group 4. Only vanadium, the lightest element, has any tendency to form compounds in oxidation states lower than +5. For example, vanadium is the only element in the group that forms stable halides in the lowest oxidation state (+2). All three metals react with excess oxygen, however, to produce the corresponding oxides in the +5 oxidation state (M2O5), in which polarization of the oxide ions by the high-oxidation-state metal is so extensive that the compounds are primarily covalent in character. Vanadium–oxygen species provide a classic example of the effect of increasing metal oxidation state on the protonation state of a coordinated water molecule: vanadium(II) in water exists as the violet hydrated ion [V(H2O)6]2+; the blue-green [V(H2O)6]3+ ion is acidic, dissociating to form small amounts of the [V(H2O)5(OH)]2+ ion and a proton; and in water, vanadium(IV) forms the blue vanadyl ion [(H2O)4VO]2+, which contains a formal V=O bond (Figure \(\PageIndex{2}\)). Consistent with its covalent character, V2O5 is acidic, dissolving in base to give the vanadate ion ([VO4]3−), whereas both Nb2O5 and Ta2O5 are comparatively inert. Oxides of these metals in lower oxidation states tend to be nonstoichiometric.
Because vanadium ions with different oxidation states have different numbers of d electrons, aqueous solutions of the ions have different colors: in acid V(V) forms the pale yellow [VO2]+ ion; V(IV) is the blue vanadyl ion [VO]2+; and V(III) and V(II) exist as the hydrated V3+ (blue-green) and V2+ (violet) ions, respectively.
Although group 5 metals react with the heavier chalcogens to form a complex set of binary chalcogenides, the most important are the dichalcogenides (MY2), whose layered structures are similar to those of the group 4 dichalcogenides. The elements of group 5 also form binary nitrides, carbides, borides, and hydrides, whose stoichiometries and properties are similar to those of the corresponding group 4 compounds. One such compound, tantalum carbide (TiC), has the highest melting point of any compound known (3738°C); it is used for the cutting edges of high-speed machine tools.
Group 6 (Cr, Mo, and W)
As an illustration of the trend toward increasing polarizability as we go from left to right across the d block, in group 6 we first encounter a metal (Mo) that occurs naturally as a sulfide ore rather than as an oxide. Molybdenite (MoS2) is a soft black mineral that can be used for writing, like PbS and graphite. Because of this similarity, people long assumed that these substances were all the same. In fact, the name molybdenum is derived from the Greek molybdos, meaning “lead.” More than 90% of the molybdenum produced annually is used to make steels for cutting tools, which retain their sharp edge even when red hot. In addition, molybdenum is the only second- or third-row transition element that is essential for humans. The major chromium ore is chromite (FeCr2O4), which is oxidized to the soluble [CrO4]2− ion under basic conditions and reduced successively to Cr2O3 and Cr with carbon and aluminum, respectively. Pure chromium can be obtained by dissolving Cr2O3 in sulfuric acid followed by electrolytic reduction; a similar process is used for electroplating metal objects to give them a bright, shiny, protective surface layer. Pure tungsten is obtained by first converting tungsten ores to WO3, which is then reduced with hydrogen to give the metal.
The metals become increasing polarizable across the d block.
Consistent with periodic trends, the group 6 metals are slightly less electropositive than those of the three preceding groups, and the two heaviest metals are essentially the same size because of the lanthanide contraction (Table \(\PageIndex{2}\)). All three elements have a total of six valence electrons, resulting in a maximum oxidation state of +6. Due to extensive polarization of the anions, compounds in the +6 oxidation state are highly covalent. As in groups 4 and 5, the lightest element exhibits variable oxidation states, ranging from Cr2+, which is a powerful reductant, to CrO3, a red solid that is a powerful oxidant. For Mo and W, the highest oxidation state (+6) is by far the most important, although compounds in the +4 and +5 oxidation states are known.
| Group | Element | Z | Valence Electron Configuration | Electronegativity | Metallic Radius (pm) | Melting Point (°C) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| 6 | Cr | 24 | 4s13d5 | 1.66 | 128 | 1907 | 7.15 |
| Mo | 42 | 5s14d5 | 2.16 | 139 | 2623 | 10.20 | |
| W | 74 | 6s25d44f14 | 1.70 | 139 | 3422 | 19.30 | |
| 7 | Mn | 25 | 4s23d5 | 1.55 | 127 | 1246 | 7.30 |
| Tc | 43 | 5s24d5 | 2.10 | 136 | 2157 | 11.50 | |
| Re | 75 | 6s25d54f14 | 1.90 | 137 | 3186 | 20.80 |
The chemistry of the two heaviest group 6 metals (Mo and W) is dominated by the +6 oxidation state. The chemistry of the lightest element (Cr) is dominated by lower oxidation states.
As observed in previous groups, the group 6 halides become more covalent as the oxidation state of the metal increases: their volatility increases, and their melting points decrease. Recall that as the electronegativity of the halogens decreases from F to I, they are less able to stabilize high oxidation states; consequently, the maximum oxidation state of the corresponding metal halides decreases. Thus all three metals form hexafluorides, but CrF6 is unstable at temperatures above −100°C, whereas MoF6 and WF6 are stable. Consistent with the trend toward increased stability of the highest oxidation state for the second- and third-row elements, the other halogens can oxidize chromium to only the trihalides, CrX3 (X is Cl, Br, or I), while molybdenum forms MoCl5, MoBr4, and MoI3, and tungsten gives WCl6, WBr5, and WI4.
Both Mo and W react with oxygen to form the covalent trioxides (MoO3 and WO3), but Cr reacts to form only the so-called sesquioxide (Cr2O3). Chromium will form CrO3, which is a highly toxic compound that can react explosively with organic materials. All the trioxides are acidic, dissolving in base to form the corresponding oxoanions ([MO4]2−). Consistent with periodic trends, the sesquioxide of the lightest element in the group (Cr2O3) is amphoteric. The aqueous chemistry of molybdate and tungstate is complex, and at low pH they form a series of polymeric anions called isopolymetallates, such as the [Mo8O26]4− ion, whose structure is as follows:
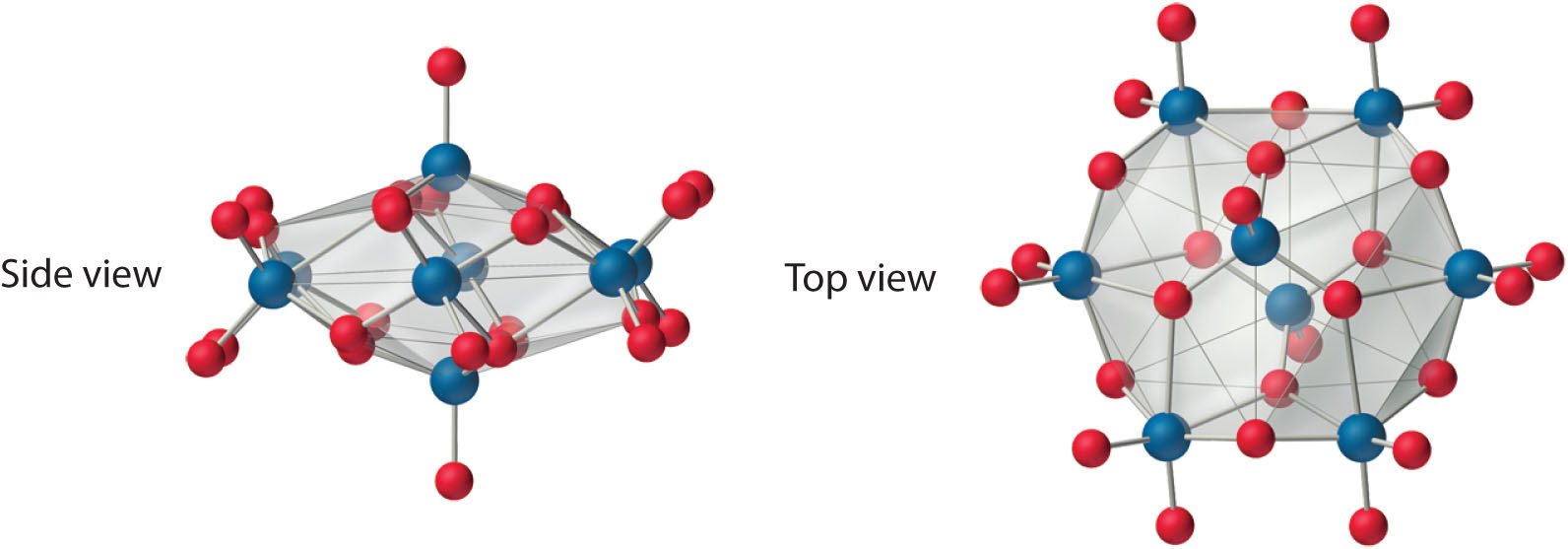
An isopolymolybdate cluster. The [Mo8O26]4− ion, shown here in both side and top views, is typical of the oxygen-bridged clusters formed by Mo(VI) and W(VI) in aqueous solution.
Reacting molybdenum or tungsten with heavier chalcogens gives binary chalcogenide phases, most of which are nonstoichiometric and electrically conducting. One of the most stable is MoS2; it has a layered structure similar to that of TiS2 (Figure \(\PageIndex{1}\)), in which the layers are held together by only weak van der Waals forces, which allows them to slide past one another rather easily. Consequently, both MoS2 and WS2 are used as lubricants in a variety of applications, including automobile engines. Because tungsten itself has an extraordinarily high melting point (3380°C), lubricants described as containing “liquid tungsten” actually contain a suspension of very small WS2 particles.
As in groups 4 and 5, the elements of group 6 form binary nitrides, carbides, and borides whose stoichiometries and properties are similar to those of the preceding groups. Tungsten carbide (WC), one of the hardest compounds known, is used to make the tips of drill bits.
Group 7 (Mn, Tc, and Re)
Continuing across the periodic table, we encounter the group 7 elements (Table \(\PageIndex{2}\)). One group 7 metal (Mn) is usually combined with iron in an alloy called ferromanganese, which has been used since 1856 to improve the mechanical properties of steel by scavenging sulfur and oxygen impurities to form MnS and MnO. Technetium is named after the Greek technikos, meaning “artificial,” because all its isotopes are radioactive. One isotope, 99mTc (m for metastable), has become an important biomedical tool for imaging internal organs. Because of its scarcity, Re is one of the most expensive elements, and its applications are limited. It is, however, used in a bimetallic Pt/Re catalyst for refining high-octane gasoline.
All three group 7 elements have seven valence electrons and can form compounds in the +7 oxidation state. Once again, the lightest element exhibits multiple oxidation states. Compounds of Mn in oxidation states ranging from −3 to +7 are known, with the most common being +2 and +4 (Figure \(\PageIndex{3}\)). In contrast, compounds of Tc and Re in the +2 oxidation state are quite rare. Because the electronegativity of Mn is anomalously low, elemental manganese is unusually reactive. In contrast, the chemistry of Tc is similar to that of Re because of their similar size and electronegativity, again a result of the lanthanide contraction. Due to the stability of the half-filled 3d5 electron configuration, the aqueous Mn3+ ion, with a 3d4 valence electron configuration, is a potent oxidant that is able to oxidize water. It is difficult to generalize about other oxidation states for Tc and Re because their stability depends dramatically on the nature of the compound.
Like vanadium, compounds of manganese in different oxidation states have different numbers of d electrons, which leads to compounds with different colors: the Mn2+(aq) ion is pale pink; Mn(OH)3, which contains Mn(III), is a dark brown solid; MnO2, which contains Mn(IV), is a black solid; and aqueous solutions of Mn(VI) and Mn(VII) contain the green manganate ion [MnO4]2− and the purple permanganate ion [MnO4]−, respectively.
Consistent with higher oxidation states being more stable for the heavier transition metals, reacting Mn with F2 gives only MnF3, a high-melting, red-purple solid, whereas Re reacts with F2 to give ReF7, a volatile, low-melting, yellow solid. Again, reaction with the less oxidizing, heavier halogens produces halides in lower oxidation states. Thus reaction with Cl2, a weaker oxidant than F2, gives MnCl2 and ReCl6. Reaction of Mn with oxygen forms only Mn3O4, a mixed-valent compound that contains two Mn(II) and one Mn(III) per formula unit and is similar in both stoichiometry and structure to magnetite (Fe3O4). In contrast, Tc and Re form high-valent oxides, the so-called heptoxides (M2O7), consistent with the increased stability of higher oxidation states for the second and third rows of transition metals. Under forced conditions, manganese will form Mn2O7, an unstable, explosive, green liquid. Also consistent with this trend, the permanganate ion [MnO4]2− is a potent oxidant, whereas [TcO4]− and [ReO4]− are much more stable. Both Tc and Re form disulfides and diselenides with layered structures analogous to that of MoS2, as well as more complex heptasulfides (M2S7). As is typical of the transition metals, the group 7 metals form binary nitrides, carbides, and borides that are generally stable at high temperatures and exhibit metallic properties.
The chemistry of the group 7 metals (Mn, Tc, and Re) is dominated by lower oxidation states. Compounds in the maximum possible oxidation state (+7) are readily reduced.
Groups 8, 9, and 10
In many older versions of the periodic table, groups 8, 9, and 10 were combined in a single group (group VIII) because the elements of these three groups exhibit many horizontal similarities in their chemistry, in addition to the similarities within each column. In part, these horizontal similarities are due to the fact that the ionization potentials of the elements, which increase slowly but steadily across the d block, have now become so large that the oxidation state corresponding to the formal loss of all valence electrons is encountered only rarely (group 8) or not at all (groups 9 and 10). As a result, the chemistry of all three groups is dominated by intermediate oxidation states, especially +2 and +3 for the first-row metals (Fe, Co, and Ni). The heavier elements of these three groups are called precious metals because they are rather rare in nature and mostly chemically inert.
The chemistry of groups 8, 9, and 10 is dominated by intermediate oxidation states such as +2 and +3.
Group 8 (Fe, Ru, and Os)
The chemistry of group 8 is dominated by iron, whose high abundance in Earth’s crust is due to the extremely high stability of its nucleus. Ruthenium and osmium, on the other hand, are extremely rare elements, with terrestrial abundances of only about 0.1 ppb and 5 ppb, respectively, and they were not discovered until the 19th century. Because of the high melting point of iron (1538°C), early humans could not use it for tools or weapons. The advanced techniques needed to work iron were first developed by the Hittite civilization in Asia Minor sometime before 2000 BC, and they remained a closely guarded secret that gave the Hittites military supremacy for almost a millennium. With the collapse of the Hittite civilization around 1200 BC, the technology became widely distributed, however, leading to the Iron Age.
Group 9 (Co, Rh, and Ir)
Cobalt is one of the least abundant of the first-row transition metals. Its oxide ores, however, have been used in glass and pottery for thousands of years to produce the brilliant color known as “cobalt blue,” and its compounds are consumed in large quantities in the paint and ceramics industries. The heavier elements of group 9 are also rare, with terrestrial abundances of less than 1 ppb; they are generally found in combination with the heavier elements of groups 8 and 10 in Ni–Cu–S ores.
Group 10 (Ni, Pd, and Pt)
Nickel silicates are easily processed; consequently, nickel has been known and used since antiquity. In fact, a 75:25 Cu:Ni alloy was used for more than 2000 years to mint “silver” coins, and the modern US nickel uses the same alloy. In contrast to nickel, palladium and platinum are rare (their terrestrial abundance is about 10–15 ppb), but they are at least an order of magnitude more abundant than the heavier elements of groups 8 and 9. Platinum and palladium are used in jewelry, the former as the pure element and the latter as the Pd/Au alloy known as white gold.

Over 2000 years ago, the Bactrian civilization in Western Asia used a 75:25 alloy of copper and nickel for its coins. A modern US nickel has the same composition, but a modern Canadian nickel is nickel-plated steel and contains only 2.5% nickel by mass.
Trends in Group 8, 9, and 10
Some properties of the elements in groups 8–10 are summarized in Table \(\PageIndex{3}\). As in earlier groups, similarities in size and electronegativity between the two heaviest members of each group result in similarities in chemistry. We are now at the point in the d block where there is no longer a clear correlation between the valence electron configuration and the preferred oxidation state. For example, all the elements of group 8 have eight valence electrons, but only Ru and Os have any tendency to form compounds in the +8 oxidation state, and those compounds are powerful oxidants. The predominant oxidation states for all three group 8 metals are +2 and +3. Although the elements of group 9 possess a total of nine valence electrons, the +9 oxidation state is unknown for these elements, and the most common oxidation states in the group are +3 and +1. Finally, the elements of group 10 all have 10 valence electrons, but all three elements are normally found in the +2 oxidation state formed by losing the ns2 valence electrons. In addition, Pd and Pt form numerous compounds and complexes in the +4 oxidation state.
| Group | Element | Z | Valence Electron Configuration | Electronegativity | Metallic Radius (pm) | Melting Point (°C) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| 8 | Fe | 26 | 4s23d6 | 1.83 | 126 | 1538 | 7.87 |
| Ru | 44 | 5s14d7 | 2.20 | 134 | 2334 | 12.10 | |
| Os | 76 | 6s25d64f14 | 2.20 | 135 | 3033 | 22.59 | |
| 9 | Co | 27 | 4s23d7 | 1.88 | 125 | 1495 | 8.86 |
| Rh | 45 | 5s14d8 | 2.28 | 134 | 1964 | 12.40 | |
| Ir | 77 | 6s25d74f14 | 2.20 | 136 | 2446 | 22.50 | |
| 10 | Ni | 28 | 4s23d8 | 1.91 | 124 | 1455 | 8.90 |
| Pd | 46 | 4d10 | 2.20 | 137 | 1555 | 12.00 | |
| Pt | 78 | 6s25d84f14 | 2.20 | 139 | 1768 | 21.50 |
We stated that higher oxidation states become less stable as we go across the d-block elements and more stable as we go down a group. Thus Fe and Co form trifluorides, but Ni forms only the difluoride NiF2. In contrast to Fe, Ru and Os form a series of fluorides up to RuF6 and OsF7. The hexafluorides of Rh and Ir are extraordinarily powerful oxidants, and Pt is the only element in group 10 that forms a hexafluoride. Similar trends are observed among the oxides. For example, Fe forms only FeO, Fe2O3, and the mixed-valent Fe3O4 (magnetite), all of which are nonstoichiometric. In contrast, Ru and Os form the dioxides (MO2) and the highly toxic, volatile, yellow tetroxides, which contain formal M=O bonds. As expected for compounds of metals in such high oxidation states, the latter are potent oxidants. The tendency of the metals to form the higher oxides decreases rapidly as we go farther across the d block.
Higher oxidation states become less stable across the d-block, but more stable down a group.
Reactivity with the heavier chalcogens is rather complex. Thus the oxidation state of Fe, Ru, Os, Co, and Ni in their disulfides is +2 because of the presence of the disulfide ion (S22−), but the disulfides of Rh, Ir, Pd, and Pt contain the metal in the +4 oxidation state together with sulfide ions (S2−). This combination of highly charged cations and easily polarized anions results in substances that are not simple ionic compounds and have significant covalent character.
The groups 8–10 metals form a range of binary nitrides, carbides, and borides. By far the most important of these is cementite (Fe3C), which is used to strengthen steel. At high temperatures, Fe3C is soluble in iron, but slow cooling causes the phases to separate and form particles of cementite, which gives a metal that retains much of its strength but is significantly less brittle than pure iron. Palladium is unusual in that it forms a binary hydride with the approximate composition PdH0.5. Because the H atoms in the metal lattice are highly mobile, thin sheets of Pd are highly permeable to H2 but essentially impermeable to all other gases, including He. Consequently, diffusion of H2 through Pd is an effective method for separating hydrogen from other gases.
Group 11 (Cu, Ag, and Au)
The coinage metals—copper, silver, and gold—occur naturally (like the gold nugget shown here); consequently, these were probably the first metals used by ancient humans. For example, decorative gold artifacts dating from the late Stone Age are known, and some gold Egyptian coins are more than 5000 yr old. Copper is almost as ancient, with objects dating to about 5000 BC. Bronze, an alloy of copper and tin that is harder than either of its constituent metals, was used before 3000 BC, giving rise to the Bronze Age. Deposits of silver are much less common than deposits of gold or copper, yet by 3000 BC, methods had been developed for recovering silver from its ores, which allowed silver coins to be widely used in ancient times.

This 1 kg gold nugget was found in Australia; in 2005, it was for sale in Hong Kong at an asking price of more than US$64,000.
Deposits of gold and copper are widespread and numerous, and for many centuries it was relatively easy to obtain large amounts of the pure elements. For example, a single gold nugget discovered in Australia in 1869 weighed more than 150 lb. Because the demand for these elements has outstripped their availability, methods have been developed to recover them economically from even very low-grade ores (as low as 1% Cu content for copper) by operating on a vast scale, as shown in the photo of an open-pit copper mine. Copper is used primarily to manufacture electric wires, but large quantities are also used to produce bronze, brass, and alloys for coins. Much of the silver made today is obtained as a by-product of the manufacture of other metals, especially Cu, Pb, and Zn. In addition to its use in jewelry and silverware, silver is used in Ag/Zn and Ag/Cd button batteries. Gold is typically found either as tiny particles of the pure metal or as gold telluride (AuTe2). It is used as a currency reserve, in jewelry, in the electronics industry for corrosion-free contacts, and, in very thin layers, as a reflective window coating that minimizes heat transfer.

The Chuquicamata copper mine in northern Chile, the world’s largest open-pit copper mine, is 4.3 km long, 3 km wide, and 825 m deep. Each gigantic truck in the foreground (and barely visible in the lower right center) can hold 330 metric tn (330,000 kg) of copper ore.
Some properties of the coinage metals are listed in Table \(\PageIndex{4}\). The electronegativity of gold (χ = 2.40) is close to that of the nonmetals sulfur and iodine, which suggests that the chemistry of gold should be somewhat unusual for a metal. The coinage metals have the highest electrical and thermal conductivities of all the metals, and they are also the most ductile and malleable. With an ns1(n − 1)d10 valence electron configuration, the chemistry of these three elements is dominated by the +1 oxidation state due to losing the single ns electron. Higher oxidation states are also known, however: +2 is common for Cu and, to a lesser extent, Ag, and +3 for Au because of the relatively low values of the second and (for Au) third ionization energies. All three elements have significant electron affinities due to the half-filled ns orbital in the neutral atoms. As a result, gold reacts with powerful reductants like Cs and solutions of the alkali metals in liquid ammonia to produce the gold anion Au− with a 6s25d10 valence electron configuration.
| Group | Element | Z | Valence Electron Configuration | Electronegativity | Metallic Radius (pm) | Melting Point (°C) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|
| 11 | Cu | 29 | 4s13d10 | 1.90 | 128 | 1085 | 8.96 |
| Ag | 47 | 5s14d10 | 1.93 | 144 | 962 | 10.50 | |
| Au | 79 | 6s15d104f14 | 2.40 | 144 | 1064 | 19.30 | |
| 12 | Zn | 30 | 4s23d10 | 1.65 | 134 | 420 | 7.13 |
| Cd | 48 | 5s24d10 | 1.69 | 149 | 321 | 8.69 | |
| Hg | 80 | 6s25d104f14 | 1.90 | 151 | −38.8 | 13.53 |
All group 11 elements are relatively unreactive, and their reactivity decreases from Cu to Au. Hence they are noble metals that are particularly well suited for use in coins and jewelry. Copper reacts with O2 at high temperatures to produce Cu2O and with sulfur to form Cu2S. Neither silver nor gold reacts directly with oxygen, although oxides of these elements can be prepared by other routes. Silver reacts with sulfur compounds to form the black Ag2S coating known as tarnish. Gold is the only metal that does not react with sulfur; it also does not react with nitrogen, carbon, or boron. All the coinage metals do, however, react with oxidizing acids. Thus both Cu and Ag dissolve in HNO3 and in hot concentrated H2SO4, while Au dissolves in the 3:1 HCl:HNO3 mixture known as aqua regia. Furthermore, all three metals dissolve in basic cyanide solutions in the presence of oxygen to form very stable [M(CN)2]− ions, a reaction that is used to separate gold from its ores.
Although the most important oxidation state for group 11 is +1, the elements are relatively unreactive, with reactivity decreasing from Cu to Au.
All the monohalides except CuF and AuF are known (including AgF). Once again, iodine is unable to stabilize the higher oxidation states (Au3+ and Cu2+). Thus all the copper(II) halides except the iodide are known, but the only dihalide of silver is AgF2. In contrast, all the gold trihalides (AuX3) are known, again except the triiodide. No binary nitrides, borides, or carbides are known for the group 11 elements.
Group 12 (Zn, Cd, and Hg)
We next encounter the group 12 elements. Because none of the elements in group 12 has a partially filled (n − 1)d subshell, they are not, strictly speaking, transition metals. Nonetheless, much of their chemistry is similar to that of the elements that immediately precede them in the d block. The group 12 metals are similar in abundance to those of group 11, and they are almost always found in combination with sulfur. Because zinc and cadmium are chemically similar, virtually all zinc ores contain significant amounts of cadmium. All three metals are commercially important, although the use of Cd is restricted because of its toxicity. Zinc is used for corrosion protection, in batteries, to make brass, and, in the form of ZnO, in the production of rubber and paints. Cadmium is used as the cathode in rechargeable NiCad batteries. Large amounts of mercury are used in the production of chlorine and NaOH by the chloralkali process, while smaller amounts are consumed in mercury-vapor streetlights and mercury batteries.
As shown in Table \(\PageIndex{4}\), the group 12 metals are significantly more electropositive than the elements of group 11, and they therefore have less noble character. They also have much lower melting and boiling points than the preceding transition metals. In contrast to trends in the preceding groups, Zn and Cd are similar to each other, but very different from the heaviest element (Hg). In particular, Zn and Cd are rather active metals, whereas mercury is not. Because mercury, the only metal that is a liquid at room temperature, can dissolve many metals by forming amalgams, medieval alchemists especially valued it when trying to transmute base metals to gold and silver. All three elements in group 12 have ns2(n − 1)d10 valence electron configurations; consequently, the +2 oxidation state, corresponding to losing the two ns electrons, dominates their chemistry. In addition, mercury forms a series of compounds in the +1 oxidation state that contain the diatomic mercurous ion Hg22+.
The most important oxidation state for group 12 is +2; the metals are significantly more electropositive than the group 11 elements, so they are less noble.
All the possible group 12 dihalides (MX2) are known, and they range from ionic (the fluorides) to highly covalent (such as HgCl2). The highly covalent character of many mercuric and mercurous halides is surprising given the large size of the cations, and this has been attributed to the existence of an easily distorted 5d10 subshell. Zinc and cadmium react with oxygen to form amphoteric MO, whereas mercury forms HgO only within a narrow temperature range (350–400°C). Whereas zinc and cadmium dissolve in mineral acids such as HCl with the evolution of hydrogen, mercury dissolves only in oxidizing acids such as HNO3 and H2SO4. All three metals react with sulfur and the other chalcogens to form the binary chalcogenides; mercury also has an extraordinarily high affinity for sulfur.
Example \(\PageIndex{1}\)
For each reaction, explain why the indicated products form.
- TiCl4(l) + 2H2O(l) → TiO2(s) + 4HCl(aq)
- WO3(s) + 3C(s) + 3Cl2(g) \(\xrightarrow{\Delta}\) WCl6(s) + 3CO(g)
- Sc2O3(s) + 2OH−(aq) + 3H2O(l) → 2[Sc(OH)4]−(aq)
- 2KMnO4(aq) + 2H2SO4(l) → Mn2O7(l) + 2KHSO4(soln) + H2O(soln)
- 4CrCl2(aq) + O2(g) + 4H+(aq) → 4Cr3+(aq) + 8Cl−(aq) + 2H2O(l)
Given: balanced chemical equation
Asked for: why the indicated products form
Strategy:
Refer to the periodic trends in this section.
Solution:
- The most stable oxidation state for Ti is +4, and neither reactant is a particularly strong oxidant or reductant; hence a redox reaction is unlikely. Similarly, neither reactant is a particularly strong acid or base, so an acid–base reaction is unlikely. Because TiCl4 contains Ti in a relatively high oxidation state (+4), however, it is likely to be rather covalent in character, with reactivity similar to that of a semimetal halide such as SiCl4. Covalent halides tend to hydrolyze in water to produce the hydrohalic acid and either the oxide of the other element or a species analogous to an oxoacid.
- This reaction involves the oxide of a group 6 metal in its highest oxidation state (WO3) and two elements, one of which is a reductant (C) and the other an oxidant (Cl2). Consequently, some sort of redox reaction will occur. Carbon can certainly react with chlorine to form CCl4, and WO3 is a potential source of oxygen atoms that can react with carbon to produce CO, which is very stable. If CO is one of the products, then it seems likely that the other product will contain the metal and chlorine. The most likely combination is WCl6 (leaving the oxidation state of the metal unchanged).
- One of the reactants is a strong base (OH−), so an acid–base reaction is likely if the other reactant is acidic. Because oxides like Sc2O3, in which the metal is in an intermediate oxidation state, are often amphoteric, we expect Sc2O3 to dissolve in base to form a soluble hydroxide complex.
- Concentrated sulfuric acid is both an oxidant and a strong acid that tends to protonate and dehydrate other substances. The permanganate ion already contains manganese in its highest possible oxidation state (+7), so it cannot be oxidized further. A redox reaction is impossible, which leaves an acid–base reaction as the most likely alternative. Sulfuric acid is likely to protonate the terminal oxygen atoms of permanganate, allowing them to be lost as water.
- Molecular oxygen is an oxidant, so a redox reaction is likely if the other reactant can be oxidized. Because chromous chloride contains chromium in its lowest accessible oxidation state, a redox reaction will occur in which Cr2+ ions are oxidized and O2 is reduced. In the presence of protons, the reduction product of O2 is water, so we need to determine only the identity of the oxidation product of Cr2+. Chromium forms compounds in two common higher oxidation states: the Cr3+ ion, which is the most stable, and the [Cr2O7]2− ion, which is a more powerful oxidant than O2. We therefore predict that the reaction will form Cr3+(aq) and water.
Exercise \(\PageIndex{1}\)
Predict the products of each reactions and then balance each chemical equation.
- Cr2+(aq) + Fe3+(aq) \(\xrightarrow{\mathrm{H^+}}\)
- Na2Cr2O7(aq) + H2SO4(l) →
- FeBr2(aq) + O2(g) \(\xrightarrow{\mathrm{H^+}}\)
- VBr4(l) + H2O(l) →
- ZrO2(s) + C(s) + Cl2(g) \(\xrightarrow{\Delta}\)
Answer:
- Cr2+(aq) + Fe3+(aq) \(\xrightarrow{\mathrm{H^+}}\) Cr3+(aq) + Fe2+(aq)
- Na2Cr2O7(aq) + 2H2SO4(l) → 2NaHSO4(soln) + H2O(soln) + 2CrO3(s)
- 4FeBr2(aq) + O2(g) + 4H+(aq) → 4Fe3+(aq) + 2H2O(l) + 8Br−(aq)
- VBr4(l) + H2O(l) → VO2+(aq) + 4Br−(aq) + 2H+(aq)
- ZrO2(s) + 2C(s) + 2Cl2(g) \(\xrightarrow{\Delta}\) ZrCl4(s) + 2CO(g)
Summary
The elements tend to become more polarizable going across the d block and higher oxidation states become less stable; higher oxidation states become more stable going down a group. The group 3 transition metals are highly electropositive metals and powerful reductants. They react with nonmetals to form compounds that are largely ionic and with oxygen to form sesquioxides (M2O3). The group 4 metals also have a high affinity for oxygen. In their reactions with halogens, the covalent character of the halides increases as the oxidation state of the metal increases because the high charge-to-radius ratio causes extensive polarization of the anions. The dichalcogenides have layered structures similar to graphite, and the hydrides, nitrides, carbides, and borides are all hard, high-melting-point solids with metallic conductivity. The group 5 metals also have a high affinity for oxygen. Consistent with periodic trends, only the lightest (vanadium) has any tendency to form compounds in oxidation states lower than +5. The oxides are sufficiently polarized to make them covalent in character. These elements also form layered chalcogenides, as well as nitrides, carbides, borides, and hydrides that are similar to those of the group 4 elements. As the metals become more polarizable across the row, their affinity for oxygen decreases. The group 6 metals are less electropositive and have a maximum oxidation state of +6, making their compounds in high oxidation states largely covalent in character. As the oxidizing strength of the halogen decreases, the maximum oxidation state of the metal also decreases. All three trioxides are acidic, but Cr2O3 is amphoteric. The chalcogenides of the group 6 metals are generally nonstoichiometric and electrically conducting, and these elements also form nitrides, carbides, and borides that are similar to those in the preceding groups. The metals of group 7 have a maximum oxidation state of +7, but the lightest element, manganese, exhibits an extensive chemistry in lower oxidation states. As with the group 6 metals, reaction with less oxidizing halogens produces metals in lower oxidation states, and disulfides and diselenides of Tc and Re have layered structures. The group 7 metals also form nitrides, carbides, and borides that are stable at high temperatures and have metallic properties. In groups 8, 9, and 10, the ionization potentials of the elements are so high that the oxidation state corresponding to the formal loss of all valence electrons is encountered rarely (group 8) or not at all (groups 9 and 10). Compounds of group 8 metals in their highest oxidation state are powerful oxidants. The reaction of metals in groups 8, 9, and 10 with the chalcogens is complex, and these elements form a range of binary nitrides, carbides, and borides. The coinage metals (group 11) have the highest electrical and thermal conductivities and are the most ductile and malleable of the metals. Although they are relatively unreactive, they form halides but not nitrides, borides, or carbides. The group 12 elements, whose chemistry is dominated by the +2 oxidation state, are almost always found in nature combined with sulfur. Mercury is the only metal that is a liquid at room temperature, and it dissolves many metals to form amalgams. The group 12 halides range from ionic to covalent. These elements form chalcogenides and have a high affinity for soft ligands.

