22.5: Oxygen
- Page ID
- 91348
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Oxygen is an element that is widely known by the general public because of the large role it plays in sustaining life. Without oxygen, animals would be unable to breathe and would consequently die. Oxygen not only is important to supporting life, but also plays an important role in many other chemical reactions. Oxygen is the most common element in the earth's crust and makes up about 20% of the air we breathe. Historically the discovery of oxygen as an element essential for combustion stands at the heart of the phlogiston controversy (see below).
The Origin and History
Oxygen is found in the group 16 elements and is considered a chalcogen. Named from the Greek oxys + genes, "acid-former", oxygen was discovered in 1772 by Scheele and independently by Priestly in 1774. Oxygen was given its name by the French scientist, Antoine Lavoisier.
Scheele discovered oxygen through an experiment which involved burning manganese oxide. Scheele came to find that the hot manganese oxide produced a gas which he called "fire air". He also came to find that when this gas was able to come into contact with charcoal, it produced beautiful bright sparks. All of the other elements produced the same gas. Although Scheele discovered oxygen, he did not publish his work until three years after another chemist, Joseph Priestly, discovered oxygen. Joseph Priestly, an English chemist, repeated Scheele's experiment in 1774 using a slightly different setup. Priestly used a 12 in burning glass and aimed the sunlight directly towards the compound that he was testing, mercuric oxide. As a result, he was able to "discover better air" that was shown to expand a mouse's lifetime to four times as long and caused a flame to burn with higher intensity. Despite these experiments, both chemists were not able to pinpoint exactly what this element was. It was not until 1775 that Antoine Lavoisier, a French chemist, was able to recognize this unknown gas as an element.
Our atmosphere currently contains about 21% of free oxygen. Oxygen is produced in various ways. The process of photochemical dissociation in which water molecules are broken up by ultraviolet rays produces about 1-2% of our oxygen. Another process that produces oxygen is photosynthesis which is performed by plants and photosynthetic bacteria. Photosynthesis occurs through the following general reaction:
\[\ce{CO2 + H2O + h\nu \rightarrow} \text{organic compounds} \ce{+ O2} \nonumber \]
Phlogiston theory is the outdated belief that a fire-like element called phlogiston is contained within combustible bodies and released during combustion. The name comes from the Ancient Greek φλογιστόν phlogistón (burning up), from φλόξ phlóx (flame). It was first stated in 1667 by Johann Joachim Becher, and then put together more formally by Georg Ernst Stahl. The theory attempted to explain burning processes such as combustion and rusting, which are now collectively known as oxidation.
Properties
- Element number: 8
- Atomic weight 15.9994
- Color: gas form- colorless, liquid- pale blue
- Melting point: 54.36K
- Boiling point: 90.2 K
- Density: .001429
- 21% of earth's atmosphere
- Third most abundant element in the universe
- Most abundant element in Earth's crust at 45.4%
- 3 Stable isotopes
- Ionization energy: 13.618 eV
- Oxygen is easily reduced and is a great oxidizing agent making it readily reactive with other elements
Magnetic Properties of Oxygen
Oxygen (O2) is paramagnetic. An oxygen molecule has six valence electrons, so the O2 molecule has 12 valence electrons with the electron configuration shown below:
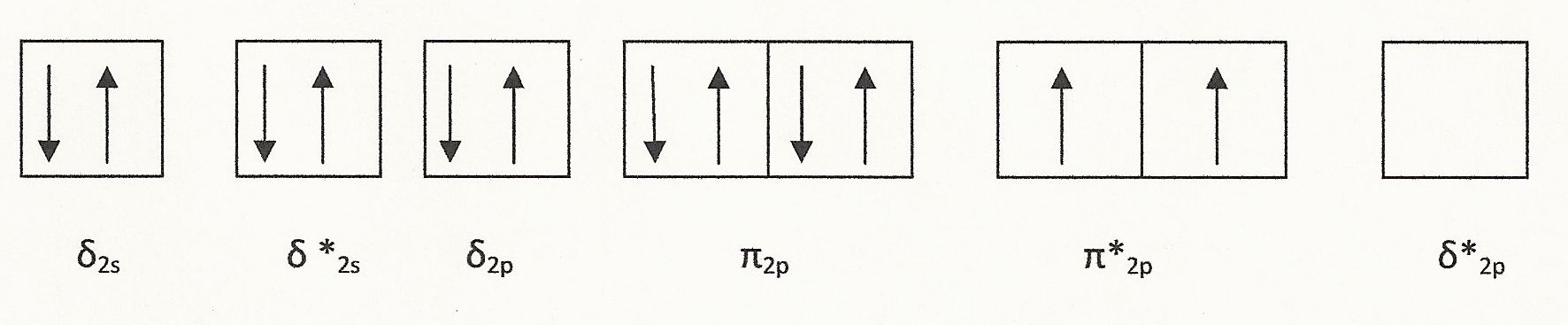
As shown, there are two unpaired electrons, which causes O2 to be paramagnetic. There are also eight valence electrons in the bonding orbitals and four in antibonding orbitals, which makes the bond order 2. This accounts for the double covalent bond that is present in O2.
Video \(\PageIndex{1}\): A chemical demonstration of the paramagnetism of molecular oxygen, as shown by the attraction of liquid oxygen to magnets.
As shown in Video \(\PageIndex{1}\), since molecular oxygen (\(O_2\)) has unpaired electrons, it is paramagnetic and is attracted to the magnet. In contrast, molecular nitrogen (\(N_2\)) has no unpaired electrons and is not attracted to the magnet.
General Chemistry of Oxygen
Oxygen normally has an oxidation state of -2, but is capable of having oxidation states of -2, -1, -1/2, 0, +1, and +2. The oxidation states of oxides, peroxides and superoxides are as follows:
- Oxides: O-2 ,
- peroxides: O2-2 ,
- superoxide: O2-1.
Oxygen does not react with itself, nitrogen, or water under normal conditions. Oxygen does, however, dissolve in water at 20 degrees Celsius and 1 atmosphere. Oxygen also does not normally react with bases or acids. Group 1 metals (alkaline metals) are very reactive with oxygen and must be stored away from oxygen in order to prevent them from becoming oxidized. The metals at the bottom of the group are more reactive than those at the top. The reactions of a few of these metals are explored in more detail below.
Lithium: Reacts with oxygen to form white lithium oxide in the reaction below.
\[\ce{4Li + O_2 \rightarrow 2Li_2O} \label{1} \]
Sodium: Reacts with oxygen to form a white mixture of sodium oxide and sodium peroxide. The reactions are shown below.
- Sodium oxide: \[\ce{4Na + O_2 \rightarrow 2Na_2O} \label{2} \]
- Sodium peroxide: \[\ce{2Na + O_2 \rightarrow Na_2O_2} \label{3} \]
Potassium: Reacts with oxygen to form a mixture of potassium peroxide and potassium superoxide. The reactions are shown below.
- Potassium peroxide: \[\ce{2K + O_2 \rightarrow 2K_2O_2} \label{4} \]
- Potassium superoxide: \[\ce{K + O_2 \rightarrow KO_2} \label{5} \]
Rubidium and Cesium: Both metals react to produce superoxides through the same process as that of the potassium superoxide reaction.
The oxides of these metals form metal hydroxides when they react with water. These metal hydroxides make the solution basic or alkaline, hence the name alkaline metals.
Group 2 metals (alkaline earth metals) react with oxygen through the process of burning to form metal oxides but there are a few exceptions.
Beryllium is very difficult to burn because it has a layer of beryllium oxide on its surface which prevents further interaction with oxygen. Strontium and barium react with oxygen to form peroxides. The reaction of barium and oxygen is shown below, and the reaction with strontium would be the same.
\[\ce{Ba(s) + O2 (g) \rightarrow BaO2 (s) }\label{6} \]
Group 13 reacts with oxygen in order to form oxides and hydroxides that are of the form \(X_2O_3\) and \(X(OH)_3\). The variable X represents the various group 13 elements. As you go down the group, the oxides and hydroxides get increasingly basic.
Group 14 elements react with oxygen to form oxides. The oxides formed at the top of the group are more acidic than those at the bottom of the group. Oxygen reacts with silicon and carbon to form silicon dioxide and carbon dioxide. Carbon is also able to react with oxygen to form carbon monoxide, which is slightly acidic. Germanium, tin, and lead react with oxygen to form monoxides and dioxides that are amphoteric, which means that they react with both acids and bases.
Group 15 elements react with oxygen to form oxides. The most important are listed below.
- Nitrogen: N2O, NO, N2O3, N2O4, N2O5
- Phosphorus: P4O6, P4O8, P2O5
- Arsenic: As2O3, As2O5
- Antimony: Sb2O3, Sb2O5
- Bismuth: Bi2O3, Bi2O5
Group 16 elements react with oxygen to form various oxides. Some of the oxides are listed below.
- Sulfur: SO, SO2, SO3, S2O7
- Selenium: SeO2, SeO3
- Tellurium: TeO, TeO2, TeO3
- Polonium: PoO, PoO2, PoO3
Group 17 elements (halogens) fluorine, chlorine, bromine, and iodine react with oxygen to form oxides. Fluorine forms two oxides with oxygen: F2O and F2O2. Both fluorine oxides are called oxygen fluorides because fluorine is the more electronegative element. One of the fluorine reactions is shown below.
\[\ce{O2 (g) + F2 (g) \rightarrow F2O2 (g)} \label{7} \]
Group 18: Some would assume that the Noble Gases would not react with oxygen. However, xenon does react with oxygen to form \(\ce{XeO_3}\) and \(\ce{XeO_4}\). The ionization energy of xenon is low enough for the electronegative oxygen atom to "steal away" electrons. Unfortunately, \(\ce{XeO_3}\) is HIGHLY unstable, and it has been known to spontaneously detonate in a clean, dry environment.
Transition metals react with oxygen to form metal oxides. However, gold, silver, and platinum do not react with oxygen. A few reactions involving transition metals are shown below:
\[2Sn_{(s)} + O_{2(g)} \rightarrow 2SnO_{(s)} \label{8} \]
\[4Fe_{(s)} + 3O_{2(g)} \rightarrow 2Fe_2O_{3(s)} \label{9A} \]
\[4Al_{(s)} + 3O_{2(g)} \rightarrow 2Al_2O_{3(s)} \label{9B} \]
Reaction of Oxides
We will be discussing metal oxides of the form \(X_2O\). The variable \(X\) represents any metal that is able to bond to oxygen to form an oxide.
- Reaction with water: The oxides react with water to form a metal hydroxide.
\[X_2O + H_2O \rightarrow 2XOH \nonumber \]
- Reaction with dilute acids: The oxides react with dilute acids to form a salt and water.
\[X_2O + 2HCl \rightarrow 2XCl + H_2O \nonumber \]
Reactions of Peroxides
The peroxides we will be discussing are of the form \(X_2O_2\). The variable \(X\) represents any metal that can form a peroxide with oxygen.
Reaction with water: If the temperature of the reaction is kept constant despite the fact that the reaction is exothermic, then the reaction proceeds as follows:
\[X_2O_2+ 2H_2O \rightarrow 2XOH + H_2O_2 \nonumber \]
If the reaction is not carried out at a constant temperature, then the reaction of the peroxide and water will result in decomposition of the hydrogen peroxide that is produced into water and oxygen.
Reaction with dilute acid: This reaction is more exothermic than that with water. The heat produced causes the hydrogen peroxide to decompose to water and oxygen. The reaction is shown below.
\[X_2O_2 + 2HCl \rightarrow 2XCl + H_2O_2 \nonumber \]
\[2H_2O_2 \rightarrow 2H_2O + O_2 \nonumber \]
Reaction of Superoxides
The superoxides we will be talking about are of the form \(XO_2\), with \(X\) representing any metal that forms a superoxide when reacting with oxygen.
Reaction with water: The superoxide and water react in a very exothermic reaction that is shown below. The heat that is produced in forming the hydrogen peroxide will cause the hydrogen peroxide to decompose to water and oxygen.
\[2XO_2 + 2H_2O \rightarrow 2XOH + H_2O_2 + O_2 \nonumber \]
Reaction with dilute acids: The superoxide and dilute acid react in a very exothermic reaction that is shown below. The heat produced will cause the hydrogen peroxide to decompose to water and oxygen.
\[2XO_2 + 2HCl \rightarrow 2XCl + H_2O_2 + O_2 \nonumber \]
Allotropes of Oxygen
There are two allotropes of oxygen; dioxygen (O2) and trioxygen (O3) which is called ozone. The reaction of converting dioxygen into ozone is very endothermic, causing it to occur rarely and only in the lower atmosphere. The reaction is shown below:
\[3O_{2 (g)} \rightarrow 2O_{3 (g)} \;\;\; ΔH^o= +285 \;kJ \nonumber \]
Ozone is unstable and quickly decomposes back to oxygen but is a great oxidizing agent.
Miscellaneous Reactions
Reaction with Alkanes: The most common reactions that involve alkanes occur with oxygen. Alkanes are able to burn and it is the process of oxidizing the hydrocarbons that makes them important as fuels. An example of an alkane reaction is the reaction of octane with oxygen as shown below.
C8H18(l) + 25/2 O2(g) → 8CO2(g) + 9H2O(l) ∆Ho = -5.48 X 103 kJ
Reaction with ammonia: Oxygen is able to react with ammonia to produce dinitrogen (N2) and water (H2O) through the reaction shown below.
\[4NH_3 + 3O_2 \rightarrow 2N_2 + 6H_2O \nonumber \]
Reaction with Nitrogen Oxide: Oxygen is able to react with nitrogen oxide in order to produce nitrogen dioxide through the reaction shown below.
\[NO + O_2 \rightarrow NO_2 \nonumber \]
Problems
- Is oxygen reactive with noble gases?
- Which transition metals does oxygen not react with?
- What is produced when an oxide reacts with water?
- Is oxygen reactive with alkali metals? Why are the alkali metals named that way?
- If oxygen is reactive with alkali metals, are oxides, peroxides or superoxides produced?
Solutions
- No, noble gases are unreactive with oxygen.
- Oxygen is mostly unreactive with gold and platinum.
- When an oxide reacts with water, a metal hydroxide is produced.
- Oxygen is very reactive with alkali metals. Alkali metals are given the name alkali because the oxides of these metals react with water to form a metal hydroxide that is basic or alkaline.
- Lithium produces an oxide, sodium produces a peroxide, and potassium, cesium, and rubidium produce superoxides.
References
- Braathen, Per Christian. "Determination of the Oxygen Content of Air ." J. Chem. Educ. 2000 77 1410.
- Najdoski, Metodija; Petrusevski, Vladimir M. " A Novel Experiment for Fast and Simple Determination of the Oxygen Content in the Air" J. Chem. Educ. 2000 77 1447.
- Petrucci, Ralph H. General Chemistry. 9th ed. Upper Saddle River: Prentice Hall, 2007. Print
- McNaught, Ian J. "A Modified Hydrogen/Oxygen Balloon Demonstration." J. Chem. Educ. 1998 75 52.
Contributors and Attributions
- Phillip Ball (UCD), Katharine Williams (UCD)

