13.E: Properties of Solutions (Exercises)
- Page ID
- 91257
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)13.1: The Solution Process
Conceptual Problems
- Classify each of the following as a heterogeneous mixture or homogeneous mixture. Explain your rationale in each case.
- aqueous ammonia
- liquid decongestant
- vinegar
- seawater
- gasoline
- fog
- Solutions and heterogeneous mixtures are at the extreme ends of the solubility scale. Name one type of mixture that is intermediate on this scale. How are the properties of the mixture you have chosen different from those of a solution or a heterogeneous mixture?
- a naphthalene mothball dissolving in benzene
- a sample of a common drain cleaner that has a mixture of \(NaOH\) crystals and \(Al\) chunks dissolving in water to give \(H_2\) gas and an aqueous solution of \(Na^+\), \(OH^−\), and \(Al^{3+}\) ions
- an iron ship anchor slowly dissolving in seawater
- sodium metal dissolving in liquid ammonia
- a sugar cube dissolving in a cup of hot tea
- \(SO_3\) gas dissolving in water to produce sulfuric acid
- calcium oxide dissolving in water to produce a basic solution
- metallic gold dissolving in a small quantity of liquid mercury
- Classify each process as simple dissolution or a chemical reaction.
- Classify each process as simple dissolution or a chemical reaction.
- You notice that a gas is evolved as you are dissolving a solid in a liquid. Will you be able to recover your original solid by evaporation? Why or why not?
- Why is heat evolved when sodium hydroxide pellets are dissolved in water? Does this process correspond to simple dissolution or a chemical reaction? Justify your answer.
- Which process(es) is the simple formation of a solution, and which process(es) involves a chemical reaction?
- mixing an aqueous solution of NaOH with an aqueous solution of HCl
- bubbling HCl gas through water
- adding iodine crystals to CCl4
- adding sodium metal to ethanol to produce sodium ethoxide (C2H5O−Na+) and hydrogen gas
- Using thermochemical arguments, explain why some substances that do not form a solution at room temperature will form a solution when heated. Explain why a solution can form even when \(ΔH_{soln}\) is positive.
- If you wanted to formulate a new compound that could be used in an instant cold pack, would you select a compound with a positive or negative value of \(ΔH_{soln}\) in water? Justify your answer.
- Why is entropy the dominant factor in the formation of solutions of two or more gases? Is it possible for two gases to be immiscible? Why or why not?
Conceptual Answers
- Homogeneous mixtures: aqueous ammonia, liquid decongestant, vinegar, and gasoline. Heterogeneous mixtures: seawater and fog.
- All are chemical reactions except dissolving iodine crystals in \(CCl_4\).
13.2: Saturated Solutions and Solubility
Conceptual Problems
- If a compound is only slightly soluble in a particular solvent, what are the relative strengths of the solvent–solvent and solute–solute interactions versus the solute–solvent interactions?
- Predict whether each of the following sets of conditions favors formation of a solution:
| Intermolecular Attractive Forces (Solute) | Intermolecular Attractive Forces (Solvent) | \(ΔH_{soln}\) |
|---|---|---|
| London dispersion | hydrogen bonding | slightly positive |
| dipole–dipole | hydrogen bonding | very negative |
| ionic | dipole–dipole | slightly positive |
| ionic | London dispersion | positive |
- Arrange the following liquids in order of increasing solubility in water: t-butanol [(CH3)3COH], benzene, ammonia, and heptane. Justify your answer.
- Which compound in each pair will be more soluble in water? Explain your reasoning in each case.
- toluene (\(C_7H_8\)) or ethyl ether (\(C_2H_5OC_2H_5\))
- chloroform (\(CHCl_3\)) or acetone (\(CH_3COCH_3\))
- carbon tetrachloride (\(CCl_4\)) or tetrahydrofuran (\(C_4H_8O\))
- \(CaCl_2\) or \(CH_2Cl_2\)
- Which compound in each pair will be more soluble in benzene? Explain your reasoning in each case.
- cyclohexane or methanol
- \(I_2\) or \(MgCl_2\)
- methylene chloride (\(CH_2Cl_2\)) or acetic acid
- Two water-insoluble compounds—n-decylamine \([CH_3(CH_2)_9NH_2]\) and n-decane—can be separated by the following procedure: The compounds are dissolved in a solvent such as toluene that is immiscible with water. When adding an aqueous \(HCl\) solution to the mixture and stirring vigorously, the \(HCl\) reacts with one of the compounds to produce a salt. When the stirring is stopped and the mixture is allowed to stand, two layers are formed. At this point, each layer contains only one of the two original compounds. After the layers are separated, adding aqueous \(NaOH\) to the aqueous layer liberates one of the original compounds, which can then be removed by stirring with a second portion of toluene to extract it from the water.
- Identify the compound that is present in each layer following the addition of HCl. Explain your reasoning.
- How can the original compounds be recovered from the toluene solution?
- Bromine and iodine are both soluble in \(CCl_4\), but bromine is much more soluble. Why?
- A solution is made by mixing 50.0 mL of liquid A with 75.0 mL of liquid B. Which is the solute, and which is the solvent? Is it valid to assume that the volume of the resulting solution will be 125 mL? Explain your answer.
- The compounds NaI, NaBr, and NaCl are far more soluble in water than NaF, a substance that is used to fluoridate drinking water. In fact, at 25°C the solubility of NaI is 184 g/100 mL of water, versus only 4.2 g/100 mL of water for NaF. Why is sodium iodide so much more soluble in water? Do you expect KCl to be more soluble or less soluble in water than NaCl?
- When water is mixed with a solvent with which it is immiscible, the two liquids usually form two separate layers. If the density of the nonaqueous solvent is 1.75 g/mL at room temperature, sketch the appearance of the heterogeneous mixture in a beaker and label which layer is which. If you were not sure of the density and the identity of the other liquid, how might you be able to identify which is the aqueous layer?
- When two liquids are immiscible, the addition of a third liquid can occasionally be used to induce the formation of a homogeneous solution containing all three.
- Ethylene glycol (\(HOCH_2CH_2OH\)) and hexane are immiscible, but adding acetone \([(CH_3)_2CO]\) produces a homogeneous solution. Why does adding a third solvent produce a homogeneous solution?
- Methanol and n-hexane are immiscible. Which of the following solvents would you add to create a homogeneous solution—water, n-butanol, or cyclohexane? Justify your choice.
- Some proponents of vitamin therapy for combating illness encourage the consumption of large amounts of fat-soluble vitamins. Why can this be dangerous? Would it be as dangerous to consume large amounts of water-soluble vitamins? Why or why not?
- Why are most metals insoluble in virtually all solvents?
- Because sodium reacts violently with water, it is difficult to weigh out small quantities of sodium metal for a reaction due to its rapid reaction with small amounts of moisture in the air. Would a Na/Hg amalgam be as sensitive to moisture as metallic sodium? Why or why not? A Na/K alloy is a liquid at room temperature. Will it be more or less sensitive to moisture than solid Na or K?
- Dental amalgams often contain high concentrations of Hg, which is highly toxic. Why isn’t dental amalgam toxic?
- Arrange 2,2,3-trimethylpentane, 1-propanol, toluene (\(C_7H_8\)), and dimethyl sulfoxide [\(\ce{(CH_3)_2S=O}\)] in order of increasing dipole moment. Explain your reasoning.
- Arrange acetone, chloroform, cyclohexane, and 2-butanol in order of increasing dielectric constant. Explain your reasoning.
- Dissolving a white crystalline compound in ethanol gave a blue solution. Evaporating the ethanol from the solution gave a bluish-crystalline product, which slowly transformed into the original white solid on standing in the air for several days. Explain what happened. How does the mass of the initial bluish solid compare with the mass of the white solid finally recovered?
- You have been asked to develop a new drug that could be used to bind Fe3+ ions in patients who suffer from iron toxicity, allowing the bound iron to be excreted in the urine. Would you consider a crown ether or a cryptand to be a reasonable candidate for such a drug? Explain your answer.
- Describe two different situations in which fractional crystallization will not work as a separation technique when attempting to isolate a single compound from a mixture.
- You have been given a mixture of two compounds—A and B—and have been told to isolate pure A. You know that pure A has a lower solubility than pure B and that the solubilities of both A and B increase with temperature. Outline a procedure to isolate pure A. If B had the lower solubility, could you use the same procedure to isolate pure A? Why or why not?
Conceptual Answers
7. London dispersion forces increase with increasing atomic mass. Iodine is a solid while bromine is a liquid due to the greater intermolecular interactions between the heavier iodine atoms. Iodine is less soluble than bromine in virtually all solvents because it requires more energy to separate \(I_2\) molecules than \(Br_2\) molecules.
11.
- A third solvent with intermediate polarity and/or dielectric constant can effectively dissolve both of the immiscible solvents, creating a single liquid phase.
- n-butanol—it is intermediate in polarity between methanol and n-hexane, while water is more polar than either and cyclohexane is comparable to n-hexane.
15. In dental amalgam, the mercury atoms are locked in a solid phase that does not undergo corrosion under physiological conditions; hence, the mercury atoms cannot readily diffuse to the surface where they could vaporize or undergo chemical reaction.
21. Dissolve the mixture of A and B in a solvent in which they are both soluble when hot and relatively insoluble when cold, filter off any undissolved B, and cool slowly. Pure A should crystallize, while B stays in solution. If B were less soluble, it would be impossible to obtain pure A by this method in a single step, because some of the less soluble compound (B) will always be present in the solid that crystallizes from solution.
13.3: Factors Affecting Solubility
Conceptual Problems
- Use the kinetic molecular theory of gases discussed in Chapter 10 to explain why the solubility of virtually all gases in liquids decreases with increasing temperature.
- An industrial plant uses water from a nearby stream to cool its reactor and returns the water to the stream after use. Over a period of time, dead fish start to appear downstream from the plant, but there is no evidence for any leaks of potentially toxic chemicals into the stream. What other factor might be causing the fish to die?
- One manufacturer’s instructions for setting up an aquarium specify that if boiled water is used, the water must be cooled to room temperature and allowed to stand overnight before fish are added. Why is it necessary for the water to stand?
- Using a carbonated beverage as an example, discuss the effect of temperature on the “fizz.” How does the “foaminess” of a carbonated beverage differ between Los Angeles, California, and Denver, Colorado?
- A common laboratory technique for degassing a solvent is to place it in a flask that is sealed to the atmosphere and then evacuate the flask to remove any gases above the liquid. Why is this procedure effective? Why does the temperature of the solvent usually decrease substantially during this process?
Conceptual Answers
- When water is boiled, all of the dissolved oxygen and nitrogen are removed. When the water is cooled to room temperature, it initially contains very little dissolved oxygen. Allowing the water to stand overnight allows oxygen in the air to dissolve, so that the fish will not suffocate.
- Evacuating the flask to remove gases decreases the partial pressure of oxygen above the solution. According to Henry’s law, the solubility of any gas decreases as its partial pressure above the solution decreases. Consequently, dissolved oxygen escapes from solution into the gas phase, where it is removed by the vacuum pump. Filling the flask with nitrogen gas and repeating this process several times effectively removes almost all of the dissolved oxygen. The temperature of the solvent decreases because some solvent evaporates as well during this process. The heat that is required to evaporate some of the liquid is initially removed from the rest of the solvent, decreasing its temperature.
Numerical Problems
- The solubility of \(CO_2\) in water at 0°C and 1 atm is 0.335 g/100 g of \(H_2O\). At 20°C and 1 atm, the solubility of \(CO_2\) in water is 0.169 g/100 g of \(H_2O\).
- What volume of \(CO_2\) would be released by warming 750 g of water saturated with \(CO_2\) from 0°C to 20°C?
- What is the value of the Henry’s law constant for \(CO_2\) under each set of conditions?
- The solubility of \(O_2\) in 100 g of \(H_2O\) at varying temperatures and a pressure of 1 atm is given in the following table:
| Solubility (g) | Temperature (°C) |
|---|---|
| 0.0069 | 0 |
| 0.0054 | 10 |
| 0.0043 | 20 |
- What is the value of the Henry’s law constant at each temperature?
- Does Henry’s law constant increase or decrease with increasing temperature?
- At what partial pressure of \(O_2\) would the concentration of \(O_2\) in water at 0°C be the same as the concentration in water at 20°C at a partial pressure of 1 atm?
- Assuming that air is 20% \(O_2\) by volume, at what atmospheric pressure would the \(O_2\) concentration be the same at 20°C as it is at atmospheric pressure and 0°C?
Numerical Answer
-
- 0.678 L \(CO_2\)
- \(k_{0°C} = 7.61 \times 10^{-2}\; M/atm\), \(k_{20°C} = 3.84 \times 10^{-2}\; M/atm\)
13.4: Ways of Expressing Concentration
Conceptual Problems
- Does the molality have the same numerical value as the molarity for a highly concentrated aqueous solution of fructose (\(C_6H_{12}O_6\)) (approximately 3.2 M)? Why or why not?
- Explain why the molality and molarity of an aqueous solution are not always numerically identical. Will the difference between the two be greater for a dilute or a concentrated solution? Explain your answer.
- Under what conditions are molality and molarity likely to be equal? Is the difference between the two greater when water is the solvent or when the solvent is not water? Why?
- What is the key difference between using mole fraction or molality versus molarity to describe the concentration of a solution? Which unit(s) of concentration is most appropriate for experiments that must be carried out at several different temperatures?
- An experiment that relies on very strict control of the reaction stoichiometry calls for adding 50.0 mL of a 0.95 M solution of A to 225 mL of a 1.01 M solution of B, followed by heating for 1 h at 60°C. To save time, a student decided to heat solution B to 60°C before measuring out 225 mL of solution B, transferring it to the flask containing solution A, and proceeding normally. This change in procedure caused the yield of product to be less than usual. How could such an apparently minor change in procedure have resulted in a decrease in the yield?
Numerical Problems
- Complete the following table for aqueous solutions of the compounds given.
| Compound | Molarity (M) | Solution Density (g/mL) | Mole Fraction (X) |
|---|---|---|---|
| \(H_2SO_4\) | 18.0 | 1.84 | |
| \(CH_3COOH\) | 1.00 | \(7.21 \times 10^{−3}\) | |
| \(KOH\) | 3.60 | 1.16 |
- Complete the following table for each compound given.
| Compound | Mass (g) | Volume of Solution (mL) | Molarity (M) |
|---|---|---|---|
| \(Na_2SO_4\) | 7.80 | 225 | |
| \(KNO_3\) | 125 | 1.27 | |
| \(NaO_2CCH_3\) | 18.64 | 0.95 |
- How would you prepare 100.0 mL of an aqueous solution with 0.40 M KI? a solution with 0.65 M \(NaCN\)?
- Calculate the molality of a solution with 775 mg of \(NaCl\) in 500.0 g of water. Do you expect the molarity to be the same as the molality? Why or why not?
- What is the molarity of each solution?
- 12.8 g of glucose (\(C_6H_{12}O_{6}\)) in water, total volume 150.0 mL
- 9.2 g of \(Na_3PO_4\) in water, total volume 200.0 mL
- 843 mg of I2 in \(EtOH\), total volume 150.0 mL
- A medication used to treat abnormal heart rhythms is labeled “Procainamide 0.5 g/250 cc.” Express this concentration in parts per thousand.
- Meperidine is a medication used for pain relief. A bottle of meperidine is labeled as 50 mg/mL. Express this concentration in parts per thousand.
- An aqueous solution that is 4.61% NaOH by mass has a density of 1.06 g/mL. Calculate the molarity of the solution, the mole fraction of \(NaOH\), and the molality of the solution.
- A solution of concentrated phosphoric acid contains 85.0% \(H_3PO_4\) by mass and has a density of 1.684 g/mL. Calculate the following.
- the molarity of the solution
- the mole fraction of \(H_3PO_4\)
- the molality of the solution
- A solution of commercial concentrated nitric acid is 16 M \(HNO3\) and has a density of 1.42 g/mL. What is the percentage of \(HNO3\) in the solution by mass? What is the molality?
- A commercial aqueous ammonia solution contains 28.0% \(NH3\) by mass and has a density of 0.899 g/mL. Calculate the following.
- the molarity
- the mole fraction
- Concentrated, or glacial, acetic acid is pure acetic acid and has a density of 1.053 g/mL. It is widely used in organic syntheses, in the manufacture of rayon and plastics, as a preservative in foods, and occasionally to treat warts. What volume of glacial acetic acid is required to prepare 5.00 L of a 1.75 M solution of acetic acid in ethanol?
- Solutions of sodium carbonate decahydrate, also known as washing soda, are used as skin cleansers. The solubility of this compound in cold water is 21.52 g/100 mL. If a saturated solution has a density of 1.20 g/mL, what is its molarity? What is the mole fraction of sodium carbonate decahydrate in this solution?
- Hydrogen peroxide (\(H_2O_2\)) is usually sold over the counter as an aqueous solution that is 3% by mass. Assuming a solution density of 1.01 g/mL, what is the molarity of hydrogen peroxide? What is the molar concentration of a solution that is 30% hydrogen peroxide by mass (density = 1.112 g/mL)? How would you prepare 100.0 mL of a 3% solution from the 30% solution?
- Determine the concentration of a solution with 825 mg of \(Na_2HPO_4\) dissolved in 450.0 mL of \(H_2O\) at 20°C in molarity, molality, mole fraction, and parts per million. Assume that the density of the solution is the same as that of water. Which unit of concentration is most convenient for calculating vapor pressure changes? Why?
- How many moles of \(Cl^−\) are there in 25.0 mL of a 0.15 M \(CaCl_2\) solution?
- How many moles of Na+ are there in 25.0 g of a \(1.33 \times 10^{-3}\; m \;Na_2HPO_4\) solution? What is the sodium concentration of this solution in ppb?
- How many grams of copper are there in 30.0 mL of a 0.100 M \(CuSO_4\) solution?
- How many grams of nitrate ion are there in 75.0 g of a \(1.75 \times 10^{−4} m\) \(Pb(NO_3)_2\) solution? What is the nitrate concentration of the solution in ppb?
- How many milliliters of a 0.750 M solution of \(K_2CrO_4\) are required to deliver 250 mg of chromate ion?
- How many milliliters of a \(1.95 \times 10^{−6} M\) solution of \(Ag_3PO_4\) are required to deliver 14.0 mg of \(Ag^+\)?
- Iron reacts with bromine according to the following equation:\[2Fe_{(s)} + 3Br_{2(aq)} \rightarrow 2FeBr_{3(aq)}\]
- How many milliliters of a \(5.0 \times 10^{−2} M\) solution of bromine in water are required to react completely with 750.0 mg of iron metal?
- Aluminum reacts with \(HCl\) according to the following equation: \[2Al_{(s)} + 6HCl_{(aq)} \rightarrow 2AlCl_{3(aq)} + 3H_{2(g)}\]
- If 25.0 mL of a solution of \(HCl\) in water is required to react completely with 1.05 g of aluminum metal, what is the molarity of the HCl solution?
- The precipitation of silver chloride is a diagnostic test for the presence of chloride ion. If 25.0 mL of 0.175 M \(AgNO_3\) are required to completely precipitate the chloride ions from 10.0 mL of an \(NaCl\) solution, what was the original concentration of \(NaCl\)?
- Barium sulfate is virtually insoluble. If a 10.0 mL solution of 0.333 M \(Ba(NO_3)_2\) is stirred with 40.0 mL of a 0.100 M \(Na_2SO_4\), how many grams of barium sulfate will precipitate? Which reactant is present in excess? What is its final concentration?
13.5: Colligative Properties
Conceptual Problems
- Why does the vapor pressure of a solvent decrease when adding a nonvolatile solute?
- Does seawater boil at the same temperature as distilled water? If not, which has the higher boiling point? Explain your answer.
- Which will be more soluble in benzene—\(O_2\) or \(HCl\)? Will \(H_2S\) or \(HCl\) be more soluble in water? Explain your reasoning in each case.
- Will the vapor pressure of a solution of hexane and heptane have an ideal vapor pressure curve (i.e., obey Raoult’s law)? Explain your answer. What properties of two liquids determine whether a solution of the two will exhibit an ideal behavior?
- Predict whether the following mixtures will exhibit negative, zero, or positive deviations from Raoult’s law. Explain your reasoning in each case.
- carbon tetrachloride and heptane
- methanol and tetrahydrofuran (C4H8O)
- acetone [(CH3)2C=CO] and dichloromethane
-
hexane and methanol
- Why are deviations from the ideal behavior predicted by Raoult’s law more common for solutions of liquids than are deviations from the ideal behavior predicted by the ideal gas law for solutions of gases?
- Boiling point elevation is proportional to the molal concentration of the solute. Is it also proportional to the molar concentration of the solution? Why or why not?
- Many packaged foods in sealed bags are cooked by placing the bag in boiling water. How could you reduce the time required to cook the contents of the bag using this cooking method?
- If the costs per kilogram of ethylene glycol and of ethanol were the same, which would be the more cost-effective antifreeze?
- Many people get thirsty after eating foods such as ice cream or potato chips that have a high sugar or salt content, respectively. Suggest an explanation for this phenomenon.
- When two aqueous solutions with identical concentrations are separated by a semipermeable membrane, no net movement of water occurs. What happens when a solute that cannot penetrate the membrane is added to one of the solutions? Why?
- A solution injected into blood vessels must have an electrolyte concentration nearly identical to that found in blood plasma. Why? What would happen if red blood cells were placed in distilled water? What would happen to red blood cells if they were placed in a solution that had twice the electrolyte concentration of blood plasma?
- If you were stranded on a desert island, why would drinking seawater lead to an increased rate of dehydration, eventually causing you to die of thirst?
- What is the relationship between the van’t Hoff factor for a compound and its lattice energy?
Numerical Problems
- Hemoglobin is the protein that is responsible for the red color of blood and for transporting oxygen from the lungs to the tissues. A solution with 11.2 mg of hemoglobin per mL has an osmotic pressure of 2.9 mmHg at 5°C. What is the molecular mass of hemoglobin?
- To determine the molar mass of the antifreeze protein from the Arctic right-eye flounder, the osmotic pressure of a solution containing 13.2 mg of protein per mL was measured and found to be 21.2 mmHg at 10°C. What is the molar mass of the protein?
- What is the osmotic pressure at 21.0°C of 13.5 mL of a solution with 1.77 g of sucrose (\)C_{12}H_{22}O_{11})\)? A solution of \(NaNO_3\) is generated by dissolving 1.25 g of NaNO3 in enough water to give a final volume of 25.0 mL. What is the osmotic pressure of this sample at 25.0°C?
- Which would have the lower vapor pressure—an aqueous solution that is 0.12 M in glucose or one that is 0.12 M in \(CaCl_2\)? Why?
- What is the total particle concentration expected for each aqueous solution? Which would produce the highest osmotic pressure?
- 0.35 M KBr
- 0.11 M MgSO4
- 0.26 M \(MgCl_2\)
- 0.24 M glucose (C6H12O6)
- The boiling point of an aqueous solution of sodium chloride is 100.37°C. What is the molality of the solution? How many grams of \(NaCl\) are present in 125 g of the solution?
- Calculate the boiling point of a solution of sugar prepared by dissolving 8.4 g of glucose (\(C_6H_{12}O_6\)) in 250 g of water.
- At 37°C, the vapor pressure of 300.0 g of water was reduced from 0.062 atm to 0.058 atm by the addition of NaBr. How many grams of NaBr were added?
- How many grams of KCl must be added to reduce the vapor pressure of 500.0 g of \(H_2O\) from 17.5 mmHg to 16.0 mmHg at 20.0°C?
- How much \(NaCl\) would you have to add to 2.0 L of water at a mountain lodge at an elevation of 7350 ft, where the pressure is 0.78 atm and the boiling point of water is 94°C, to get the water to boil at the same temperature as in New Orleans, Louisiana, where the pressure is 1.00 atm?
- You have three solutions with the following compositions: 12.5 g of KCl in 250 mL of water, 12.5 g of glucose in 400 mL of water, and 12.5 g of \(MgCl_2\) in 350 mL of water. Which will have the highest boiling point?
- Assuming the price per kilogram is the same, which is a better salt to use for deicing wintry roads—\(NaCl\) or \(MgCl_2\)? Why? Would magnesium chloride be an effective deicer at a temperature of −8°C?
- How many grams of \(KNO_3\) must be added to water to produce the same boiling point elevation as a solution of 2.03 g of \(MgCl_2\) in a total volume of 120.0 mL of solution, assuming complete dissociation? If the van’t Hoff factor for \(MgCl_2\) at this concentration is 2.73, how much \(KNO_3\) would be needed?
- Calculate the quantity of each compound that would need to be added to lower the freezing point of 500.0 mL of water by 1.0°C: KBr, ethylene glycol, \(MgBr_2\), ethanol. Assume that the density of water is 1.00 g/cm3.
- The melting point depression of biphenyl (melting point = 69.0°C) can be used to determine the molecular mass of organic compounds. A mixture of 100.0 g of biphenyl and 2.67 g of naphthalene (\(C_{10}H_8\)) has a melting point of 68.50°C. If a mixture of 1.00 g of an unknown compound with 100.0 g of biphenyl has a melting point of 68.86°C, what is the molar mass of the unknown compound?
- Four solutions of urea in water were prepared, with concentrations of 0.32 m, 0.55 m, 1.52 m, and 3.16 m. The freezing points of these solutions were found to be −0.595°C, −1.02°C, −2.72°C, and −5.71°C, respectively. Graphically determine the freezing point depression constant for water. A fifth solution made by dissolving 6.22 g of urea in 250.0 g of water has a freezing point of −0.75°C. Use these data to determine the molar mass of urea.
- The term osmolarity has been used to describe the total solute concentration of a solution (generally water), where 1 osmole (Osm) is equal to 1 mol of an ideal, nonionizing molecule.
- What is the osmolarity of a 1.5 M solution of glucose? a 1.5 M solution of \(NaCl\)? a 1.5 M solution of \(CaCl_2\)?
- What is the relationship between osmolarity and the concentration of water?
- What would be the direction of flow of water through a semipermeable membrane separating a 0.1 M solution of \(NaCl\) and a 0.1 M solution of \(CaCl_2\)?
- At 40°C, the vapor pressures of pure \(CCl_4\) and cyclohexane are 0.2807 atm and 0.2429 atm, respectively. Assuming ideal behavior, what is the vapor pressure of a solution with a \(CCl_4\) mole fraction of 0.475? What is the mole fraction of cyclohexane in the vapor phase? The boiling points of \(CCl_4\) and cyclohexane are 76.8°C and 80.7°C, respectively.
- A benzene/toluene solution with a mole fraction of benzene of 0.6589 boils at 88°C at 1 atm. The vapor pressures of pure benzene and toluene at this temperature are 1.259 atm and 0.4993 atm, respectively. What is the composition of the vapor above the boiling solution at this temperature?
- Plot the vapor pressure of the solution versus composition for the system \(CCl_4:CH_3CN\) at 45°C, given the following experimental data:
| \(X_{CCl_4}(liquid)\) | 0.035 | 0.375 | 0.605 | 0.961 |
|---|---|---|---|---|
| \(X_{CCl_4}(vapor)\) | 0.180 | 0.543 | 0.594 | 0.800 |
| Total P (atm) | 0.326 | 0.480 | 0.488 | 0.414 |
|
||||
- Does your diagram show behavior characteristic of an ideal solution? Explain your answer.
Numerical Answers
1. \(6.7 \times 10^4\; amu\)
3. 9.24 atm
5. The \(CaCl_2\) solution will have a lower vapor pressure, because it contains three times as many particles as the glucose solution.
7. 0.36 m \(NaCl\), 2.6 g \(NaCl\)
9. 60 g NaBr
11. 700 g \(NaCl\)
13. \(MgCl_2\) produces three particles in solution versus two for \(NaCl\), so the same molal concentration of \(MgCl_2\) will produce a 50% greater freezing point depression than for \(NaCl\). Nonetheless, the molar mass of \(MgCl_2\) is 95.3 g/mol versus 48.45 g/mol for \(NaCl\). Consequently, a solution containing 1 g \(NaCl\) per 1000 g \(H_2O\) will produce a freezing point depression of 0.064°C versus 0.059°C for a solution containing 1 g \(MgCl_2\) per 1000 g \(H_2O\). Thus, given equal cost per gram, \(NaCl\) is more effective. Yes, \(MgCl_2\) would be effective at −8°C; a 1.43 m solution (136 g per 1000 g H2O) would be required.
16.
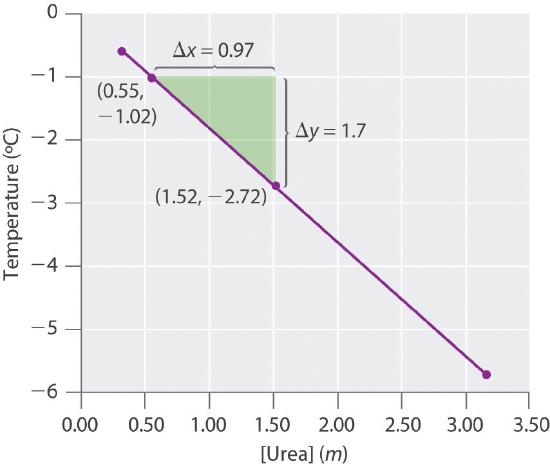
kf = 1.81(°C•kg)/mol, molecular mass of urea = 60.0 g/mol
13.6: Colloids
Conceptual Problems
- How does a colloid differ from a suspension? Which has a greater effect on solvent properties, such as vapor pressure?
- Is homogenized milk a colloid or a suspension? Is human plasma a colloid or a suspension? Justify your answers.
- How would you separate the components of an emulsion of fat dispersed in an aqueous solution of sodium chloride?

