14.2: Lipids and Triglycerides
- Page ID
- 58857
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Outcomes
- Identify the basic structural features of fatty acids.
- Distinguish between saturated and unsaturated fatty acids.
- Define essential fatty acids.
- Identify the structural features of a triglyceride.
- Label the type of bond formed between fatty acids and glycerol in triglyceride.
There is a lot of interest these days on healthy diets as well as concerns about heart problems. There is also a strong market for the sales of omega-3 fatty acids, which are said to help lower fat levels in blood. But too many people rely on the supplements to help their hearts and don't understand the chemistry behind it all. Yes, taking omega-3 fatty acids will give you some of the fatty acids your body requires. No, this is not a substitute for eating a healthy diet and exercising. You can't sit in front of the TV set, eating your large pizza, and expect these pills to keep you health. You've got to do things the hard way - eat your vegetables and get some exercise.
Fatty Acids
A lipid is an organic compound such as fat or oil. Organisms use lipids to store energy, but lipids have other important roles as well. Lipids consist of repeating units called fatty acids. Fatty acids are organic compounds that have the general formula \(\ce{CH_3(CH_2)_{n}COOH}\), where \(n\) usually ranges from 2 to 28 and is always an even number. There are two types of fatty acids: saturated fatty acids and unsaturated fatty acids.
Saturated Fatty Acids
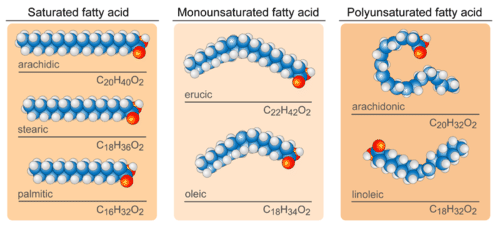
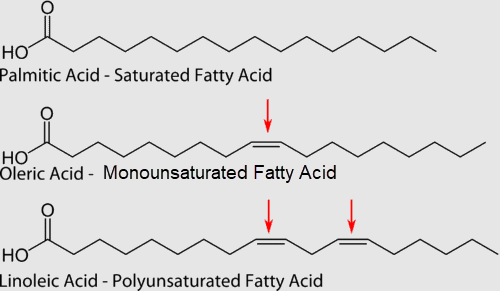
In saturated fatty acids, carbon atoms are bonded to as many hydrogen atoms as possible. This causes the molecules to form straight chains, as shown in the figure below. The straight chains can be packed together very tightly, allowing them to store energy in a compact form. This explains why saturated fatty acids are solids at room temperature. Animals use saturated fatty acids to store energy.
Unsaturated Fatty Acids
In unsaturated fatty acids, some carbon atoms are not bonded to as many hydrogen atoms as possible due to the presence of one or more double bonds in the carbon chain. Instead, they are bonded to other groups of atoms. Wherever carbon binds with these other groups of atoms, it causes chains to bend (see figure above). The bent chains cannot be packed together very tightly, so unsaturated fatty acids are liquids at room temperature. Plants use unsaturated fatty acids to store energy.
Lipids and Diet
Unsaturated fat is generally considered to be healthier because it contains fewer calories than an equivalent amount of saturated fat. Additionally, high consumption of saturated fats is linked to an increased risk of cardiovascular disease. Some examples of foods with high concentrations of saturated fats include butter, cheese, lard, and some fatty meats. Foods with higher concentrations of unsaturated fats include nuts, avocado, and vegetable oils such as canola oil and olive oil.
Humans need lipids for many vital functions, such as storing energy and forming cell membranes. Lipids can also supply cells with energy. In fact, a gram of lipids supplies more than twice as much energy as a gram of carbohydrates or proteins. Lipids are necessary in the diet for most of these functions. Although the human body can manufacture most of the lipids it needs, there are others, called essential fatty acids, that must be consumed in food. Essential fatty acids include omega-3 and omega-6 fatty acids. Both of these fatty acids are needed for important biological processes, not just for energy.
Although some lipids in the diet are essential, excess dietary lipids can be harmful. Because lipids are very high in energy, eating too many may lead to unhealthy weight gain. A high-fat diet may also increase lipid levels in the blood. This, in turn, can increase the risk for health problems such as cardiovascular disease. The dietary lipids of most concern are saturated fatty acids, trans fats, and cholesterol. For example, cholesterol is the lipid mainly responsible for narrowing arteries and causing the disease atherosclerosis.
Types of Lipids
Lipids may consist of fatty acids alone, or they may contain other molecules as well. For example, some lipids contain alcohol or phosphate groups. They include
- triglycerides: the main form of stored energy in animals.
- phospholipids: the major components of cell membranes.
- steroids: serve as chemical messengers and have other roles.
Triglycerides
One type of lipid is called a triglyceride, an ester derived from glycerol combined with three fatty acid molecules.
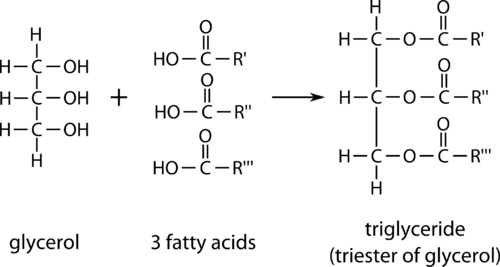
Glycerol is a triol, an alcohol which contains three hydroxyl functional groups. A fatty acid is a long carbon chain, generally from 12 to 24 carbons in length, with an attached carboxyl group. Each of the three fatty acid molecules undergoes an esterification with one of the hydroxyl groups of the glycerol molecule. The result is a large triester molecule referred to as a triglyceride.

Triglycerides function as a long-term storage form of energy in the human bods. Because of the long carbon chains, triglycerides are nearly nonpolar molecules and thus do not dissolve readily in polar solvents such as water. Instead, oils and fats are soluble in nonpolar organic solvents such as hexane and ethers.
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)

