14.E: Biological Molecules (Exercises)
- Page ID
- 59423
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)These are homework exercises to accompany Chapter 14 of the University of Kentucky's LibreText for CHE 103 - Chemistry for Allied Health. Answers are below the questions.
Questions
(click here for solutions)
Q14.1.1
What do enzymes do in the body?
Q14.1.2
Describe each of the following.
- active site
- substrate
- allosteric site
- inhibitor
Q14.1.3
How are competitive and noncompetitive inhibitors similar and different from one another?
Q14.1.4
An inhibitor interacts with the enzyme at an allosteric site. Is it competitive or noncompetitive?
Q14.1.5
Describe each of the following enzyme-substrate pairs as using the lock-and-key model or the induced fit model.

Q14.1.6
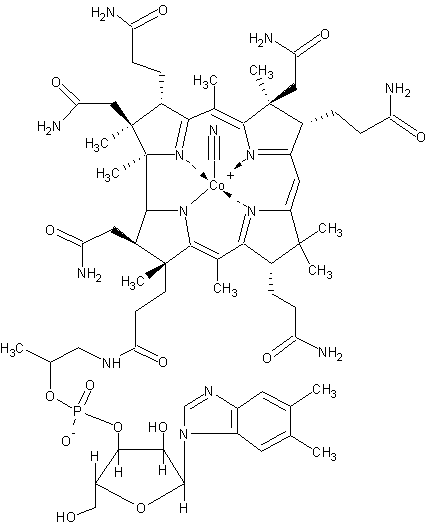
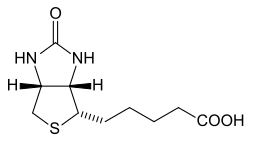
Each of the following behaves as a cofactor or a coenzyme. Identify each.
- Zn2+
- Vitamin B12
- biotin
- Fe3+
|
Vitamin B12 |
biotin |
Q14.1.7
Identify each statement as true or false. Correct the false statement(s).
- Enzyme activity increases with temperature.
- Enzyme activity depends on pH.
- Enzymes are consumed in a chemical reaction.
- Increasing the concentration will increase the rate of a reaction until all of the enzyme is "occupied".
- Enzymes can only function at their ideal temperature and pH.
Q14.1.8
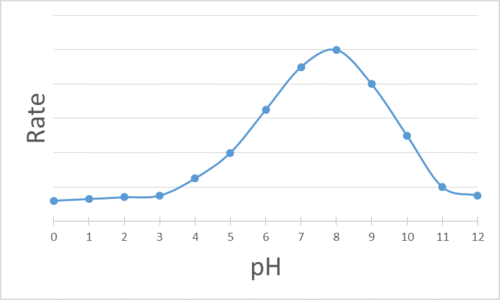
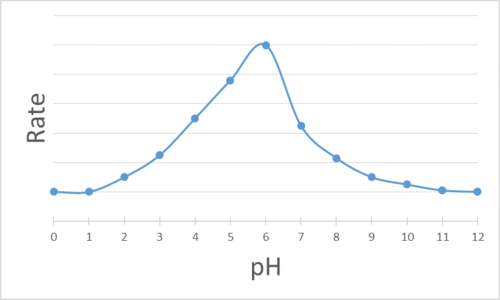
The enzyme activity is graphed with respect to the pH of the mixture. Determine the pH at which each enzyme is most effective.
(click here for solutions)
Q14.2.1
What is the functional group in a fatty acid?
Q14.2.2
What is the difference between a fat and an oil?
Q14.2.3
Butter is a fat that is a solid at room temperature. What type of fatty acid does butter contain? How do you know?
Q14.2.4
Describe the difference between saturated and unsaturated fatty acids.
Q14.2.5
Explain why molecules of saturated and unsaturated fatty acids have different shapes.
Q14.2.6
Draw each structure.
- Saturated fatty acid with 18 carbon atoms.
- Monounsaturated fatty acid with 14 carbon atoms.
- Polyunsaturated fatty acid with 14 carbon atoms.
- Monounsaturated with 16 carbon atoms.
- Polyunsaturated fatty acid with 18 carbon atoms and three double bonds.
Q14.2.7
Where does the body get essential fatty acids?
Q14.2.8
What molecules react to form a triglyceride?
Q14.2.9
Draw a triglyceride formed from three identical fatty acids.
Q14.2.10
Draw a triglyceride formed from three different fatty acids.
(click here for solutions)
Q14.3.1
What is a phospholipid?
Q14.3.2
Which part of the phospholipid molecule is water-soluble?
Q14.3.3
Which part is not water-soluble?
Q14.3.4
What is the purpose of a semipermeable membrane like the cell membrane?
Answers
14.1: Enzymes
Q14.1.1
Enzymes act as catalysts for biological reactions.
Q14.1.2
Describe each of the following.
- The active site is where the reaction occurs in the enzyme.
- The substrate is the molecule that interacts with the enzyme.
- An allosteric site is on the enzyme away from the active site. Inhibitors can interactive with the enzyme at the allosteric site.
- An inhibitor is a molecule that interacts with the molecule to slow or stop a reaction.
Q14.1.3
Both competitive and noncompetitive inhibitors slow or stop a reaction. Competitive inhibitors bind with the active site and non-competitive inhibitors bind with an allosteric site.
Q14.1.4
noncompeititve
Q14.1.5
- lock & key
- lock & key
- induced fit
Q14.1.6
- cofactor
- coenzyme
- coenzyme
- cofactor
Q14.1.7
Identify each statement as true or false. Correct the false statement(s).
- Enzyme activity increases with temperature and then decreases with increasing temperature beyond a peak temperature.
- True
- Enzymes are not consumed in a chemical reaction.
- True
- Enzymes
Q14.1.8
- pH = 8
- pH = 6
14.2: Lipids and Triglycerides
Q14.2.1
carboxylic acid
Q14.2.2
Fats are solids at room temperatures while oils are liquids.
Q14.2.3
Saturated fatty acid.
Q14.2.4
Saturated fatty acids contain only single bonds between carbon atoms while unsaturated fatty acids contain one or more double bonds.
Q14.2.5
Saturated fatty acids have a straight chain of carbon atoms while unsaturated fatty acids have a bend at every double bond.
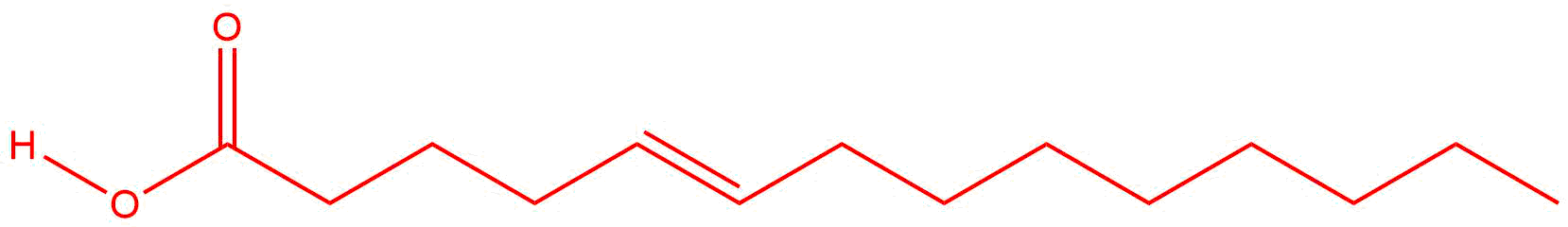
Q14.2.6
| a. | 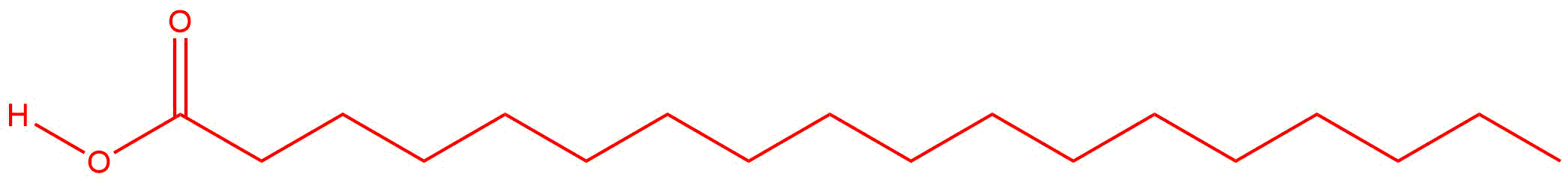 |
| b. |
Answers will vary. The double bond may be located between any two carbon atoms.
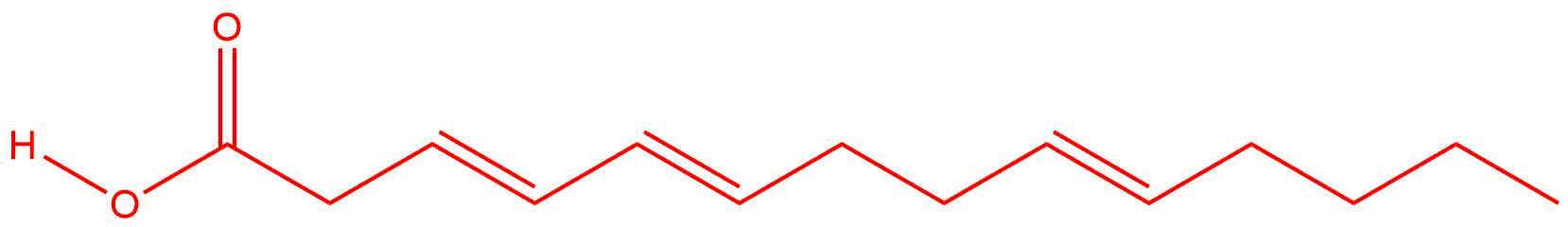
|
| c. |
Answers will vary. There should be multiple double bonds and can be located between any pairs of carbon atoms.
|
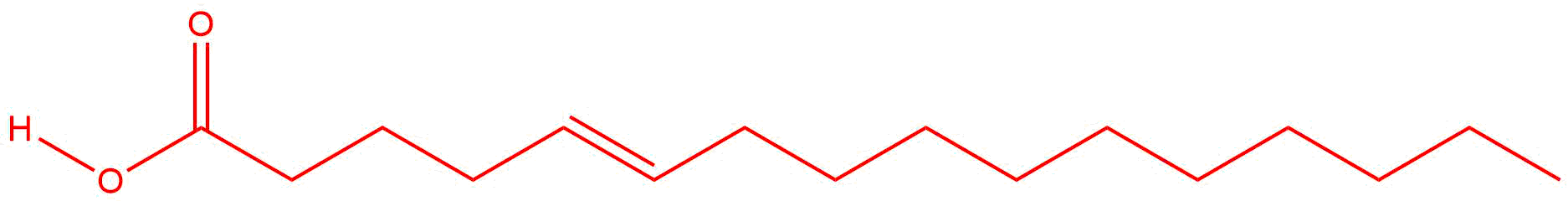
| d. |
Answers will vary. The double bond may be located between any two carbon atoms.
|
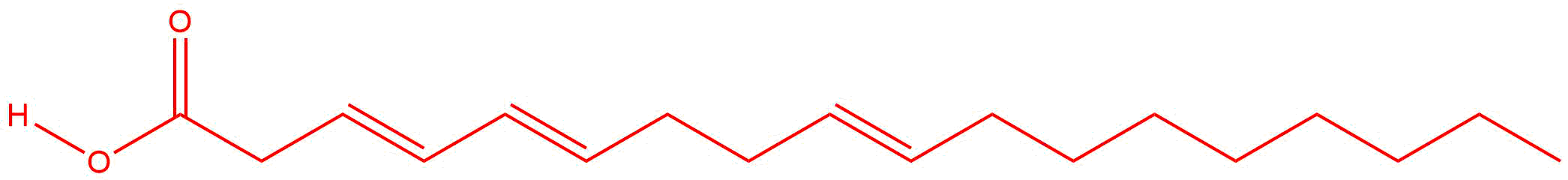
| e. |
Answers will vary. There should be three double bonds and can be located between any pairs of carbon atoms.
|
Q14.2.7
Essential fatty acids come from the food we eat.
Q14.2.8
Glycerol and three fatty acids from a triglyceride.
Q14.2.9
Answers will vary but each "tail" should have the same number of carbon atoms.
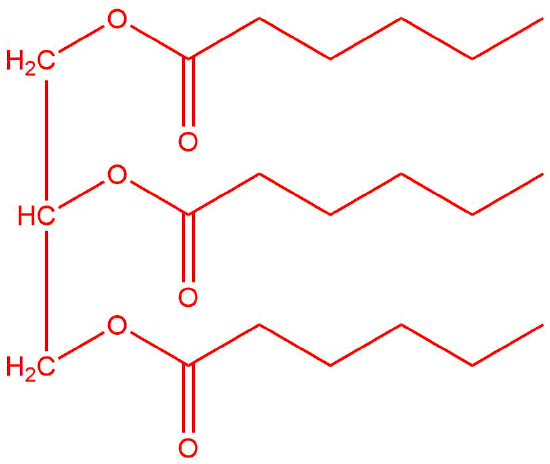
Q14.2.10
Answers will vary but each "tail" should have a different number of carbon atoms.
14.3: Phospholipids in Cell Membranes
Q14.3.1
A phospholipid is a triglyceride where one of the fatty acid tails is replaced by a phosphate group.
Q14.3.2
The phosphate end is water-soluble.
Q14.3.3
The hydrocarbon tails are not water-soluble.
Q14.3.4
To control the flow of substances in and out of the cell.