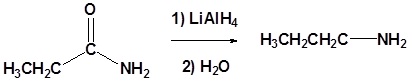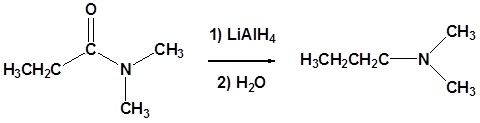20.7: Reduction of Carboxylic Acids and Their Derivatives
- Page ID
- 28263
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Since relatively few methods exist for the reduction of carboxylic acid derivatives to aldehydes, it would be useful to modify the reactivity and solubility of LAH to permit partial reductions of this kind to be achieved. The most fruitful approach to this end has been to attach alkoxy or alkyl groups on the aluminum. This not only modifies the reactivity of the reagent as a hydride donor, but also increases its solubility in nonpolar solvents. Two such reagents will be mentioned here; the reactive hydride atom is colored blue.
![]() Lithium tri-tert-butoxyaluminohydride (LtBAH), LiAl[OC(CH3)3]3H : Soluble in THF, diglyme & ether.
Lithium tri-tert-butoxyaluminohydride (LtBAH), LiAl[OC(CH3)3]3H : Soluble in THF, diglyme & ether.
![]() Diisobutylaluminum hydride (DIBAH), [(CH3)2CHCH2]2AlH : Soluble in toluene, THF & ether.
Diisobutylaluminum hydride (DIBAH), [(CH3)2CHCH2]2AlH : Soluble in toluene, THF & ether.
Each of these reagents carries one equivalent of hydride. The first (LtBAH) is a complex metal hydride, but the second is simply an alkyl derivative of aluminum hydride. In practice, both reagents are used in equimolar amounts, and usually at temperatures well below 0 ºC. The following examples illustrate how aldehydes may be prepared from carboxylic acid derivatives by careful application of these reagents. A temperature of -78 ºC is easily maintained by using dry-ice as a coolant.
Reduction of Acid Chlorides and Esters
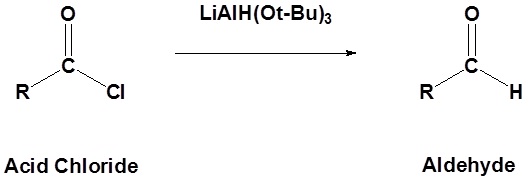
Acid chlorides can be converted to aldehydes using lithium tri-tert-butoxyaluminum hydride (LiAlH(Ot-Bu)3). The hydride source (LiAlH(Ot-Bu)3) is a weaker reducing agent than lithium aluminum hydride. Because acid chlorides are highly activated they still react with the hydride source; however, the formed aldehyde will react slowly, which allows for its isolation.
General Reaction:
| Example 1 |
|---|
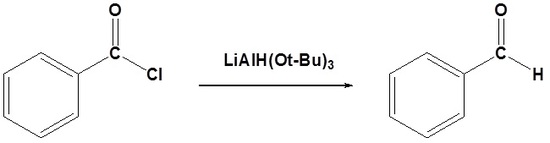 |
Acid chlorides can be converted to aldehydes using lithium tri-tert-butoxyaluminum hydride (LiAlH(Ot-Bu)3). The hydride source (LiAlH(Ot-Bu)3) is a weaker reducing agent than lithium aluminum hydride. Because acid chlorides are highly activated they still react with the hydride source; however, the formed aldehyde will react slowly, which allows for its isolation.
General Reaction:

| Example 1 |
|---|
 |
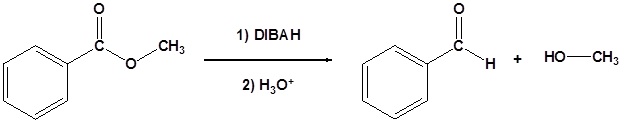
Esters can be converted to aldehydes using diisobutylaluminum hydride (DIBAH). The reaction is usually carried out at -78 oC to prevent reaction with the aldehyde product.
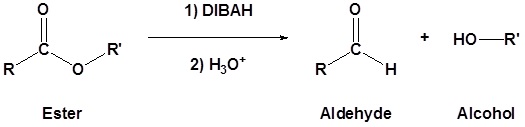
| Example 1: |
|---|
|
|
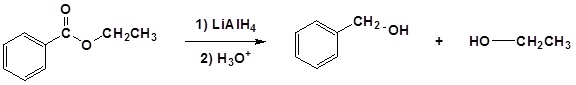
Esters can be converted to 1o alcohols using LiAlH4, while sodium borohydride (\(NaBH_4\)) is not a strong enough reducing agent to perform this reaction.
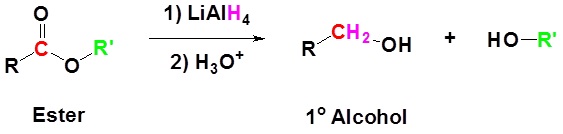
| Example 1: |
|---|
 |
Mechanism
1) Nucleophilic attack by the hydride
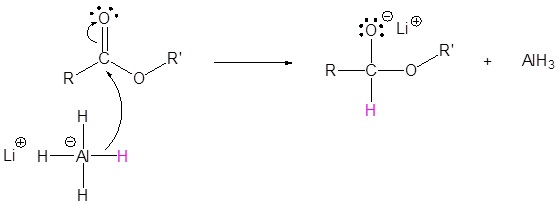
2) Leaving group removal
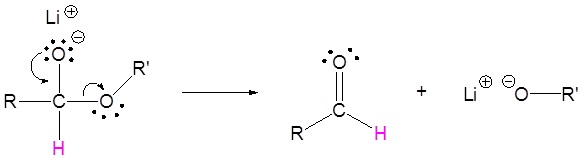
3) Nucleopilic attack by the hydride anion
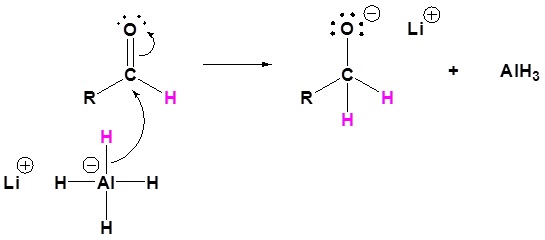
4) The alkoxide is protonated
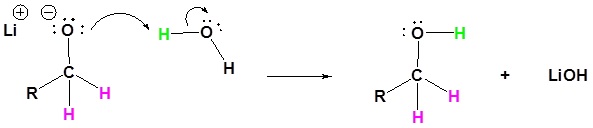
Reduction of Carboxylic Acids and Amides
Carboxylic acids can be converted to 1o alcohols using Lithium aluminum hydride (LiAlH4). Note that NaBH4 is not strong enough to convert carboxylic acids or esters to alcohols. An aldehyde is produced as an intermediate during this reaction, but it cannot be isolated because it is more reactive than the original carboxylic acid.
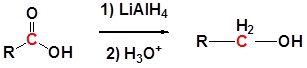
Going from reactant to products simplified
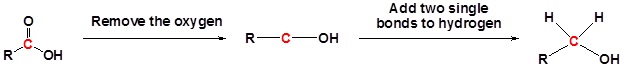
Example
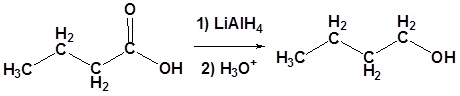
Possible Mechanism
1) Deprotonation
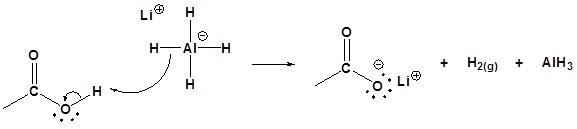
2) Nucleopilic attack by the hydride anion
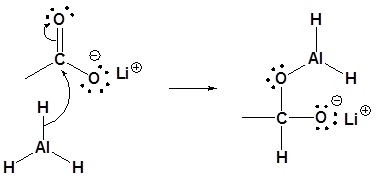
3) Leaving group removal
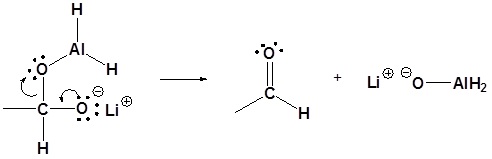
4) Nucleopilic attack by the hydride anion
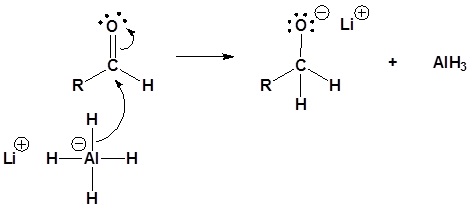
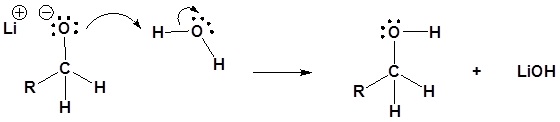
5) The alkoxide is protonated
Amides can be converted to 1°, 2° or 3° amines using LiAlH4.
General Reaction
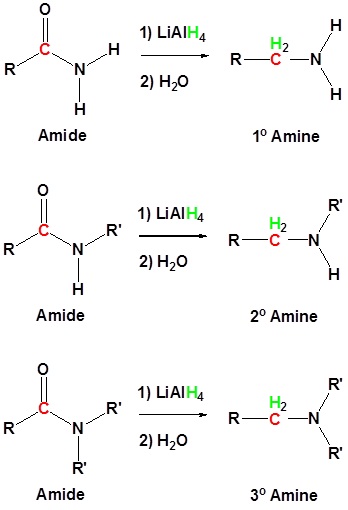
| Example 1: Amide Reductions |
|---|
|
Alkyl groups attached to the nitrogen do not affect the reaction.
|
Mechanism
1) Nucleophilic attach by the hydride
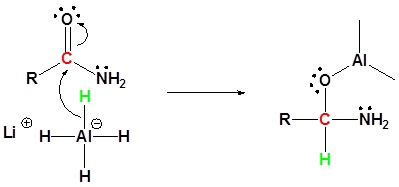
2) Leaving group removal
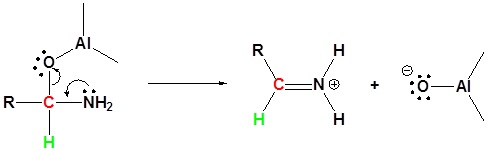
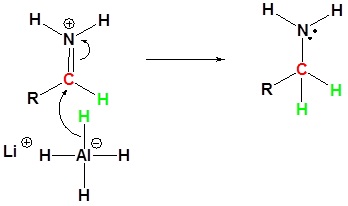
3) Nucleophilic attach by the hydride
Contributors
Prof. Steven Farmer (Sonoma State University)