6.5: Enthalpy and Entropy
- Page ID
- 28158
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Enthalpy
Thermodynamics is the study of the relationship between heat (or energy) and work. Enthalpy is a central factor in thermodynamics. It is the heat content of a system. The heat that passes into or out of the system during a reaction is the enthalpy change. Whether the enthalpy of the system increases (i.e. when energy is added) or decreases (because energy is given off) is a crucial factor that determines whether a reaction can happen.
Sometimes, we call the energy of the molecules undergoing change the "internal enthalpy". Sometimes, we call it the "enthalpy of the system." These two phrases refer to the same thing. Similarly, the energy of the molecules that do not take part in the reaction is called the "external enthalpy" or the "enthalpy of the surroundings".
Roughly speaking, the energy changes that we looked at in the introduction to thermodynamics were changes in enthalpy. We will see in the next section that there is another energetic factor, entropy, that we also need to consider in reactions. For now, we will just look at enthalpy.
- Enthalpy is the heat content of a system.
- The enthalpy change of a reaction is roughly equivalent to the amount of energy lost or gained during the reaction.
- A reaction is favored if the enthalpy of the system decreases over the reaction.
That last statement is a lot like the description of energetics on the previous page. If a system undergoes a reaction and gives off energy, its own energy content decreases. It has less energy left over if it gave some away. Why does the energy of a set of molecules change when a reaction occurs? To answer that, we need to think about what happens in a chemical reaction.
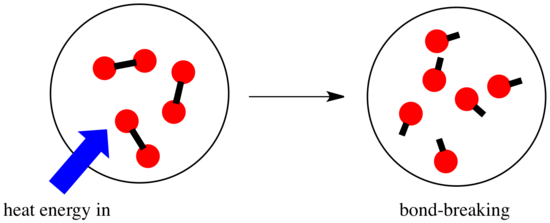
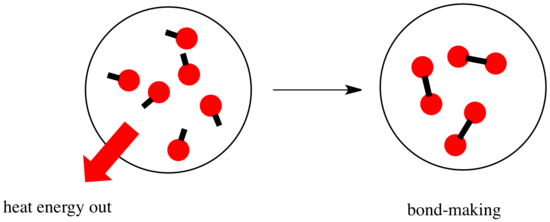
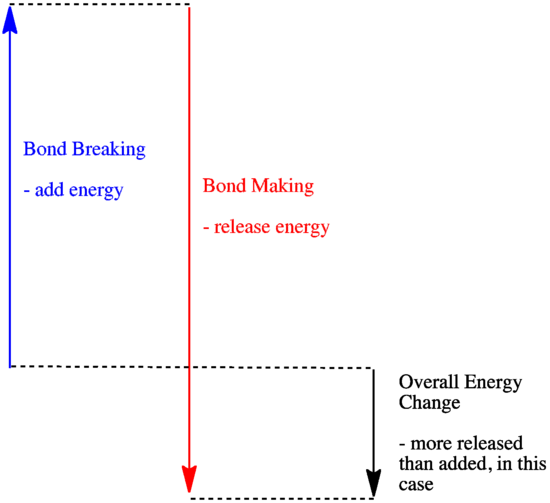
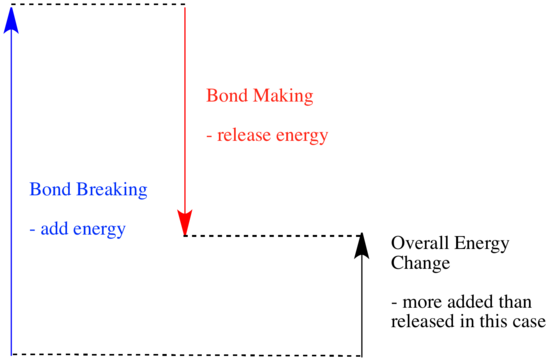
In a reaction, there is a change in chemical bonding. Some of the bonds in the reactants are broken, and new bonds are made to form the products. It costs energy to break bonds, but energy is released when new bonds are made.
Whether a reaction is able to go forward may depend on the balance between these bond-making and bond-breaking steps.
- A reaction is exothermic if more energy is released by formation of new bonds than is consumed by breaking old bonds.
- A reaction is exothermic if weaker bonds are traded for stronger ones.
- A reaction is endothermic if bond-breaking costs more energy than what is provided in bond-making.
Bond energies (the amount of energy that must be added in order to break a bond) are an important factor in determining whether a reaction will occur. Bond strengths are not always easy to predict, because the strength of a bond depends on a number of factors. However, lots of people have done lots of work measuring bond strengths, and they have collected the information in tables, so if you need to know how strong a bond is, you can just look up the information you need.
| Bond | Bond Energy (kcal/mol) | Bond | Bond Energy (kcal/mol) |
| H-H | 104 | O-H | 111 |
| C-C | 83 | C-H | 99 |
| O=O | 119 | N-H | 93 |
| N=N | 226 | C=O | 180 |
For example, suppose you wanted to know whether the combustion of methane were an exothermic or endothermic reaction. I am going to guess that it's exothermic, because this reaction (and others like it) is used to provide heat for lots of homes by burning natural gas in furnaces.
The "combustion" of methane means that it is burned in air, so that it reacts with oxygen. The products of burning hydrocarbons are mostly carbon dioxide and water. The carbon atom in methane (CH4) gets incorporated into a carbon dioxide molecule. The hydrogen atoms get incorporated into water molecules. There are four hydrogen atoms in methane, so that's enough to make two molecules of H2O.
- Four C-H bonds must be broken in the combustion of methane.
- Four new O-H bonds are made when the hydrogens from methane are added into new water molecules.
- Two new C=O bonds are made when the carbon from methane is added into a CO2 molecule.
The other piece of the puzzle is the oxygen source for the reaction. Oxygen is present in the atmosphere mostly as O2. Because we need two oxygen atoms in the CO2 molecule and two more oxygen atoms for the two water molecules, we need a total of four oxygen atoms for the reaction, which could be provided by two O2 molecules.
-
Two O=O bonds must be broken to provide the oxygen atoms for the products.
Altogether, that's four C-H and two O=O bonds broken, plus two C=O and four O-H bonds made. That's 4 x 99 kcal/mol for the C-H bonds and 2 x 119 kcal/mol for the O=O bonds, a total of 634 kJ/mol added. The reaction releases 2 x 180 kcal/mol for the C=O bonds and 4 x 111 kcla/mol for the OH bonds, totaling 804 kcal/mol. Overall, there is 170 kcal/mol more released than is consumed.
That means the reaction is exothermic, so it produces heat. It's probably a good way to heat your home.
Entropy
Observations of natural processes led a surprising number of chemists of the late 19th century (including Berthelot and Thomsen) to conclude that all spontaneous reactions must be exothermic since:
- Objects roll downhill spontaneously (i.e., energy is "lost" from the system)
- Objects do not roll uphill spontaneously (i.e., energy does not suddenly appear from nowhere)
If this were true all we would need to predict whether a reaction is spontaneous is the change of enthalpy. If \(ΔH\) were negative, the process should be able to occur by itself. If ΔH were positive, the reaction could not occur by itself.
Indeed, almost all exothermic reactions are spontaneous at standard thermodynamic conditions (1 atmosphere pressure) and \(25^oC\). However a number of common processes which are both endothermic and spontaneous are known. The most obvious are simple phase changes, like ice melting at room temperature. Also, many solids dissolve in water and simultaneously absorb heat.
So the energy is now dispersed among the molecules of liquid water which have access to all kinds of molecular motion states that were not available in the solid. At the same time, the ordered structure of the solid ice has given way to a much less organized flowing liquid.
But the actual change has occurred in the energy dispersal. This subtle property that matter possesses in terms of the way energy is dispersed in it is known as entropy. Entropy is sometimes erroneously referred to as "randomness" or even "disorder" but these descriptions do not fit the state of energy as well as they seem to describe some of the often obvious results.
Just as reactions which form stronger bonds tend to occur spontaneously, energy is constantly being dispersed or "spread out" in any process which either happens on its own or which we make happen.
It is the Second Law of Thermodynamics which gives us the criterion we are seeking to decide whether a reaction will be spontaneous or not (well, almost...):
In a spontaneous process the entropy of the universe increases.
The "universe" is a pretty big place. Recall the definitions we used when we introduced chemical thermodynamics. The system is that part of the universe on which we focus our attention--generally chemicals. The surroundings are everything else. Taken together they constitute the universe.
So the Second Law could be written this way for a spontaneous process:
Entropy trends and physical properties
(values in )
1. Entropy increases with mass
- \(F_{2 (g)}\) = 203 J/mol·K
- \(Cl_{2 (g)}\) = 224 J/mol·K
- \(Br_{2\;(g)}\) = 245 J/mol·K
- \(I_{2\; (g)}\) = 261 J/mol·K
2. Entropy increases with melting, vaporization or sublimation
-
\(I_{2(s)}\) = 117 J/mol·K vs. \(I_{2(ℓ)}\) = 261 J/mol·K and
-
\(H_2O_{(ℓ)}\) = 70 J/mol·K vs. \(H_2O_{(g)}\) = 189 J/mol·K
3. Entropy increases when solids or liquids dissolve in water
-
CH3OH(ℓ) = 127 J/mol·K vs. \(CH_3OH_{(aq)}\) = 132 J/mol·K and
-
NaCl(s) = 72J/mol·K vs. Na+(aq) + Cl-(aq) = 115 J/mol·K
4. Entropy decreases when a gas is dissolved in water
- \(HCl_{(g)}\) = 187 vs. \(H^+_{(aq)}\) + \(Cl^-_{(aq)}\) = 55
5. Entropy is lower in hard, brittle substances than in malleable solids like metals
- Diamond (C) = 2.4J/mol·K vs. Pb = 65 J/mol·K
6. Entropy increases with chemical complexity
- \(NaCl\) = 72 J/mol·K vs. \(MgCl_2\) = 90 J/mol·K vs. \(AlCl_3\) = 167 J/mol·K
Of course, the main issue here is how entropy changes during a process. This can be determined by calculation from standard entropy values (\(S^o\)) in the same way that enthalpy changes are calculated:
- Layne A. Morsch (University of Illinois Springfield)