1.5: Resonance
- Page ID
- 28099
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Objective
After completing this section, you should be able to
- draw resonance forms for molecules and ions.
Key Terms
Make certain that you can define, and use in context, the key term below.
- resonance form
- delocalization
Resonance Delocalization
Sometimes, even when formal charges are considered, the bonding in some molecules or ions cannot be described by a single Lewis structure. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. A molecule or ion with such delocalized electrons is represented by several contributing structures (also called resonance contributors or canonical forms). Resonance contributors involve the ‘imaginary movement’ of pi-bonded electrons or of lone-pair electrons that are adjacent to pi bonds. Note, sigma bonds cannot be broken during resonance – if you show a sigma bond forming or breaking, you are showing a chemical reaction taking place. Likewise, the positions of atoms in the molecule cannot change between resonance contributors.
When looking at the structure of the molecule, formate, we see that there are two equivalent structures possible. Which one is correct? There are two simple answers to this question: 'both' and 'neither one'. Both ways of drawing the molecule are equally acceptable approximations of the bonding picture for the molecule, but neither one, by itself, is an accurate picture of the delocalized pi bonds. The two alternative drawings, however, when considered together, give a much more accurate picture than either one on its own. This is because they imply, together, that the carbon-carbon bonds are not double bonds, not single bonds, but about halfway in between.
Formate Ion
Structures are Equivalent in Energy
When it is possible to draw more than one valid structure for a compound or ion, we have identified resonance contributors: two or more different Lewis structures depicting the same molecule or ion that, when considered together, do a better job of approximating delocalized pi-bonding than any single structure. By convention, resonance contributors are linked by a double-headed arrow, and are sometimes enclosed by brackets:
The depiction of formate using the two resonance contributors A and B in the figure above does not imply that the molecule at one moment looks like structure A, then at the next moment shifts to look like structure B. Rather, at all moments, the molecule is a combination, or resonance hybrid of both A and B. Each individual resonance contributor of the formate ion is drawn with one carbon-oxygen double bond (120 pm) and one carbon-oxygen single bond (135 pm), with a negative formal charge located on the single-bonded oxygen. However, the two carbon-oxygen bonds in formate are actually the same length (127 pm) which implies that neither resonance contributor is correct. Although there is an overall negative formal charge on the formate ion, it is shared equally between the two oxygens. Therefore, the formate ion can be more accurately depicted by a pair of resonance contributors. Alternatively, a single structure can be used, with a dashed line depicting the resonance-delocalized pi bond and the negative charge located in between the two oxygens.
The electrostatic potential map of the similar ion acetate shows that there is an equal amount of electron density (shown in red) around each oxygen (which is the same for formate).
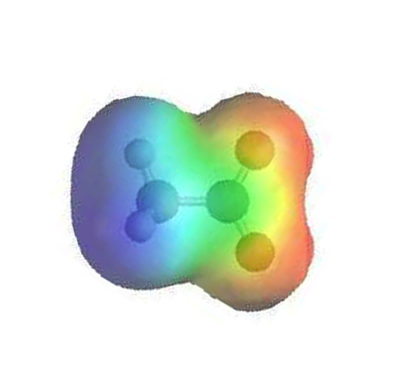
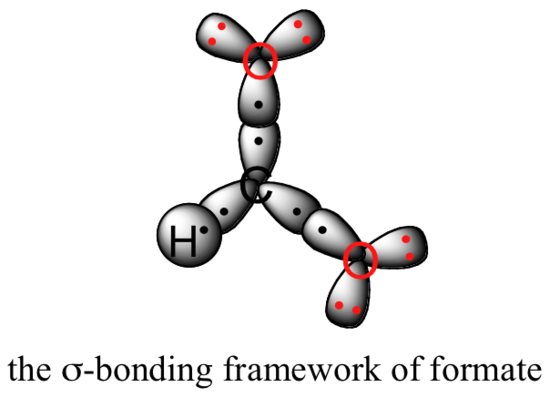
Valence bond theory can be used to develop a picture of the bonding in a carboxylate group. We know that the carbon must be sp2-hybridized, (the bond angles are close to 120˚, and the molecule is planar), and we will treat both oxygens as being sp2-hybridized as well. Both carbon-oxygen sigma bonds, then, are formed from the overlap of carbon sp2 orbitals and oxygen sp2 orbitals.
In addition, the carbon and both oxygens each have an un-hybridized 2pz orbital situated perpendicular to the plane of the sigma bonds. These three 2pz orbitals are parallel to each other, and can overlap in a side-by-side fashion to form a delocalized pi bond.
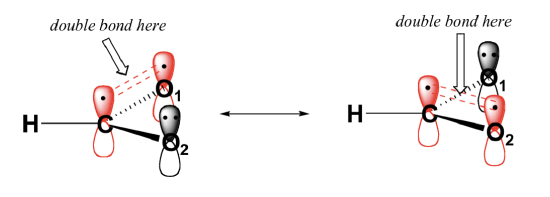
Overall, the situation is one of three parallel, overlapping 2pz orbitals sharing four delocalized pi electrons. Because there is one more electron than there are 2pz orbitals, the system has an overall charge of 1–. Resonance contributors are used to approximate overlapping 2pz orbitals and delocalized pi electrons. Molecules with resonance are usually drawn showing only one resonance contributor for the sake of simplicity. However, identifying molecules with resonance is an important skill in organic chemistry.
This example shows an important exception to the general rules for determining the hybridization of an atom. The oxygen with the negative charge appears to be sp3 hybridized because it is surrounded by four electron groups. However, this representation of the oxygen atom is not correct because it is actually part of a resonance hybrid. A pair of lone pair of electrons on the negatively charged oxygen are not localized in an sp3 orbital, rather, they are delocalized as part of a conjugated pi system. The stability gained though resonance is enough to cause the expected sp3 to become sp2. The sp2 hybridization gives the oxygen a p orbital allowing it to participate in conjugation. As a general rule sp3 hybridized atoms with lone pair electrons tend to become sp2 hybridized when adjacent to a conjugated system.
Example: Carbonate (CO32−)
Like formate, the electronic structure of the carbonate ion cannot be described by a single Lewis electron structure. Unlike O3, though, the Lewis structures describing CO32− has three equivalent representations.![]()
1. Because carbon is the least electronegative element, we place it in the central position:
2. Carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are 2 more for the 2− charge. This gives 4 + (3 × 6) + 2 = 24 valence electrons.
3. Six electrons are used to form three bonding pairs between the oxygen atoms and the carbon:
4. We divide the remaining 18 electrons equally among the three oxygen atoms by placing three lone pairs on each and indicating the 2− charge:
5. There are no electrons left for the central atom.![]()
6. At this point, the carbon atom has only 6 valence electrons, so we must take one lone pair from an oxygen and use it to form a carbon–oxygen double bond. In this case, however, there are three possible choices:
As with formate, none of these structures describes the bonding exactly. Each predicts one carbon–oxygen double bond and two carbon–oxygen single bonds, but experimentally all C–O bond lengths are identical. We can write resonance structures (in this case, three of them) for the carbonate ion:
As the case for formate, the actual structure involves the formation of a molecular orbital from pz orbitals centered on each atom and sitting above and below the plane of the CO32− ion. ![]()
Example 1.5.1
Benzene is a common organic solvent that was previously used in gasoline; it is no longer used for this purpose, however, because it is now known to be a carcinogen. The benzene molecule (C6H6) consists of a regular hexagon of carbon atoms, each of which is also bonded to a hydrogen atom. Use resonance structures to describe the bonding in benzene.
Given: molecular formula and molecular geometry
Asked for: resonance structures
Strategy:
A Draw a structure for benzene illustrating the bonded atoms. Then calculate the number of valence electrons used in this drawing.
B Subtract this number from the total number of valence electrons in benzene and then locate the remaining electrons such that each atom in the structure reaches an octet.
C Draw the resonance structures for benzene.
Solution:
A Each hydrogen atom contributes 1 valence electron, and each carbon atom contributes 4 valence electrons, for a total of (6 × 1) + (6 × 4) = 30 valence electrons. If we place a single bonding electron pair between each pair of carbon atoms and between each carbon and a hydrogen atom, we obtain the following:
Each carbon atom in this structure has only 6 electrons and has a formal charge of 1+, but we have used only 24 of the 30 valence electrons.
B If the 6 remaining electrons are uniformly distributed pair-wise on alternate carbon atoms, we obtain the following:
Three carbon atoms now have an octet configuration and a formal charge of 1−, while three carbon atoms have only 6 electrons and a formal charge of 1+. We can convert each lone pair to a bonding electron pair, which gives each atom an octet of electrons and a formal charge of 0, by making three C=C double bonds.
C There are, however, two ways to do this:
Each structure has alternating double and single bonds, but experimentation shows that each carbon–carbon bond in benzene is identical, with bond lengths (139.9 pm) intermediate between those typically found for a C–C single bond (154 pm) and a C=C double bond (134 pm). We can describe the bonding in benzene using the two resonance structures, but the actual electronic structure is an average of the two. The existence of multiple resonance structures for aromatic hydrocarbons like benzene is often indicated by drawing either a circle or dashed lines inside the hexagon:
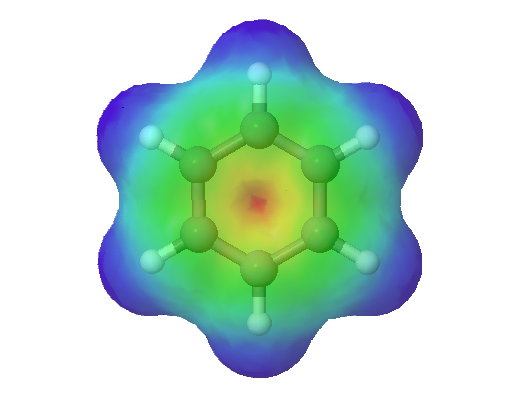
This combination of p orbitals for benzene can be visualized as a ring with a node in the plane of the carbon atoms. As can be seen in an electrostatic potential map of benzene, the electrons are distributed symmetrically around the ring.
Exercise \(\PageIndex{1}\)
Rules for Drawing and Working with Resonance Contributors
Recognizing, drawing, and evaluating the relative stability of resonance contributors is essential to understanding organic reaction mechanisms. When learning to draw and interpret resonance structures, there are a few basic guidelines to help. .
1) There is ONLY ONE REAL STRUCTURE for each molecule or ion. This real structure (the resonance hybrid) takes its character from the average of all the individual resonance contributors. When looking at a resonance contributors, we are seeing the exact same molecule or ion depicted in different ways. Resonance hybrids are really a single, unchanging structure.
Major resonance contributors of the formate ion
Representations of the formate resonance hybrid
2) The resonance hybrid is more stable than any individual resonance structures. Often, resonance structures represent the movement of a charge between two or more atoms. The charge is spread out amongst these atoms and therefore more stabilized. When looking at the picture above the resonance contributors represent the negative charge as being on one oxygen or the other. The resonance hybrid shows the negative charge being shared equally between two oxygens. In the resonance hybrid, the negative charge is spread out over a larger part of the molecule and is therefore more stable.
3) Resonance contributors do not have to be equivalent. Because of this, resonance structures do necessarily contribute equally to the resonance hybrid. The two resonance structures shown below are not equivalent because one show the negative charge on an oxygen while the other shows it on a carbon. Later, we will show that the contributor with the negative charge on the oxygen is the more stable of the two. Also, this means that the resonance hybrid will not be an exact mixture of the two structures.
4) All resonance contributors must be correct Lewis structures. Each atom should have a complete valence shell and be shown with correct formal charges. A carbocation (carbon with only 6 valence electrons) is the only allowed exception to the valence shell rules. The structure below is an invalid resonance structure even though it only shows the movement of a pi bond. The resulting structure contains a carbon with ten electrons, which violates the octet rule, making it invalid.
5) All resonance contributors must have the same molecular formula, the same number of electrons, and same net charge. The molecules in the figure below are not resonance structures of the same molecule because then have different molecular formulas (C2H5NO Vs. C2H6NO). Also, the two structures have different net charges (neutral Vs. positive).
6) Resonance contributors only differ by the positions of pi bond and lone pair electrons. Sigma bonds are never broken or made, because of this atoms must maintain their same position. The molecules in the figure below are not resonance structures of the same molecule even though they have the same molecular formula (C3H6O). These molecules are considered structural isomers because their difference involves the breaking of a sigma bond and moving a hydrogen atom.
Major and Minor Resonance Contributors
As previously state the true structure of a resonance hybrid is the combination of all the possible resonance structures. If the resonance structures are equal in stability they the contribute equally to the structure of the hybrid. However, if the resonance structures have different stabilities they contribute to the hybrid's structure in proportions related to their relative stabilities. It can be said the the resonance hybrid's structure resembles the most stable resonance structure. Because of this it is important to be able to compare the stabilities of resonance structures. In the example below, structure B is much less important in terms of its contribution to the hybrid because it contains the violated octet of a carbocation. The relative stabilities of the two structures are so vastly different that molecules which contain a C=O bond are almost exclusively written in a form like structure A. However, as will learn in chapter 19, the positively charged carbon created by structure B will explain how the C=O bond will react with electron rich species.
Rules for Estimating Stability of Resonance Structures
1. The resonance structures in which all atoms have complete valence shells is more stable. This means most atoms have a full octet. In the example below structure A has a carbon atom with a positive charge and therefore an incomplete octet. Based on this criterion, structure A is less stable and is a more minor contributor to the resonance hybrid than structure B.
2. The structures with the least number of formal charges is more stable. Based on this, structure B is less stable because is has two atoms with formal charges while structure A has none. Structure A would be the major resonance contributor.
3. The structures with a negative charge on the more electronegative atom will be more stable. The difference between the two resonance structures is the placement of a negative charge. Structure B is the more stable and the major resonance contributor, because it places the negative charge on the more electronegative oxygen.
4. The structures with a positive charges on the least electronegative atom (most electropositive) is more stable.
5. The structures with the least separation of formal charges is more stable. The only difference between the two structures below are the relative positions of the positive and negative charges. In structure A the charges are closer together making it more stable.
6. Resonance forms that are equivalent have no difference in stability. When looking at the two structures below no difference can be made using the rules listed above. This means the two structures are equivalent in stability and would make equal structural contributions to the resonance hybrid.
Examples of major and minor contributors
Example 1:
Example 2:
Example 3:
Carboxylate example
In the case of carboxylates, contributors A and B below are equivalent in terms of their relative contribution to the hybrid structure. However, there is also a third resonance contributor C, in which the carbon bears a positive formal charge (a carbocation) and both oxygens are single-bonded and bear negative charges.
Structure C makes a less important contribution to the overall bonding picture of the group relative to A and B. How do we know that structure C is the ‘minor’ contributor? Apply the rules below
- The carbon in contributor C does not have an octet. In general, resonance contributors in which a carbon does not fulfill the octet rule are relatively less important. (rule #1)
- In structure C, there are only three bonds, compared to four in A and B. In general, a resonance structure with a lower number of total bonds is relatively less important. (rule #2)
- Structure C also has more formal charges than are present in A or B. In general, resonance contributors in which there is more/greater separation of charge are relatively less important. (rule #3)
- Structures A and B are equivalent and will be equal contributors to the resonance hybrid. (rule #5).
- The resonance contributor in which a negative formal charge is located on a more electronegative atom, usually oxygen or nitrogen, is more stable than one in which the negative charge is located on a less electronegative atom such as carbon. An example is in the upper left expression in the next figure. (rule #4)
Example \(\PageIndex{1}\)
Draw the major resonance contributor of the structure below. Include in your figure the appropriate curved arrows showing how you got from the given structure to your structure. Explain why your contributor is the major one. In what kind of orbitals are the two lone pairs on the oxygen?
Solution
In the structure above, the carbon with the positive formal charge does not have a complete octet of valence electrons. Using the curved arrow convention, a lone pair on the oxygen can be moved to the adjacent bond to the left, and the electrons in the double bond shifted over to the left (see the rules for drawing resonance contributors to convince yourself that these are 'legal' moves).
The resulting resonance contributor, in which the oxygen bears the formal charge, is the major one because all atoms have a complete octet, and there is one additional bond drawn (resonance rules #1 and #2 both apply). This system can be thought of as four parallel 2p orbitals (one each on C2, C3, and C4, plus one on oxygen) sharing four pi electrons. One lone pair on the oxygen is in an unhybridized 2p orbital and is part of the conjugated pi system, and the other is located in an sp2 orbital.
Also note that one additional contributor can be drawn, but it is also minor because it has a carbon with an incomplete octet:
Contributors
- Layne Morsch (University of Illinois Springfield)
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- Dr. Krista Cunningham



