Conceptual Problems
Q10.1.1
Explain the differences between the microscopic and the macroscopic properties of matter. Is the boiling point of a compound a microscopic or macroscopic property? molecular mass? Why?
Q10.1.2
How do the microscopic properties of matter influence the macroscopic properties? Can you relate molecular mass to boiling point? Why or why not?
Q10.1.3
For a substance that has gas, liquid, and solid phases, arrange these phases in order of increasing density.
- strength of intermolecular interactions.
- compressibility.
- molecular motion.
- order in the arrangement of the molecules or atoms.
Q10.1.4
Which elements of the periodic table exist as gases at room temperature and pressure? Of these, which are diatomic molecules and which are monatomic? Which elements are liquids at room temperature and pressure? Which portion of the periodic table contains elements whose binary hydrides are most likely gases at room temperature?
Q10.2.1
What four quantities must be known to completely describe a sample of a gas? What units are commonly used for each quantity?
Q10.2.2
If the applied force is constant, how does the pressure exerted by an object change as the area on which the force is exerted decreases? In the real world, how does this relationship apply to the ease of driving a small nail versus a large nail?
Q10.2.3
As the force on a fixed area increases, does the pressure increase or decrease? With this in mind, would you expect a heavy person to need smaller or larger snowshoes than a lighter person? Explain.
Q10.2.4
What do we mean by barometric pressure? Is the barometric pressure at the summit of Mt. Rainier greater than or less than the pressure in Miami, Florida? Why?
Q10.2.5
Which has the highest barometric pressure—a cave in the Himalayas, a mine in South Africa, or a beach house in Florida? Which has the lowest?
Q10.2.6
Mars has an average barometric pressure of 0.007 atm. Would it be easier or harder to drink liquid from a straw on Mars than on Earth? Explain your answer.
Q10.2.7
Is the pressure exerted by a 1.0 kg mass on a 2.0 m2 area greater than or less than the pressure exerted by a 1.0 kg mass on a 1.0 m2 area? What is the difference, if any, between the pressure of the atmosphere exerted on a 1.0 m2 piston and a 2.0 m2 piston?
Q10.2.8
If you used water in a barometer instead of mercury, what would be the major difference in the instrument?
Numerical Problems
Q10.2.9
Calculate the pressure in pascals and in atmospheres exerted by a carton of milk that weighs 1.5 kg and has a base of 7.0 cm × 7.0 cm. If the carton were lying on its side (height = 25 cm), would it exert more or less pressure? Explain your reasoning.
Q10.2.10
If barometric pressure at sea level is 1.0 × 105 Pa, what is the mass of air in kilograms above a 1.0 cm2 area of your skin as you lie on the beach? If barometric pressure is 8.2 × 104 Pa on a mountaintop, what is the mass of air in kilograms above a 4.0 cm2 patch of skin?
Q10.2.11
Complete the following table:
Q10.2.12
The SI unit of pressure is the pascal, which is equal to 1 N/m2. Show how the product of the mass of an object and the acceleration due to gravity result in a force that, when exerted on a given area, leads to a pressure in the correct SI units. What mass in kilograms applied to a 1.0 cm2 area is required to produce a pressure of
- 1.0 atm?
- 1.0 torr?
- 1 mmHg?
- 1 kPa?
Q10.2.13
If you constructed a manometer to measure gas pressures over the range 0.60–1.40 atm using the liquids given in the following table, how tall a column would you need for each liquid? The density of mercury is 13.5 g/cm3. Based on your results, explain why mercury is still used in barometers, despite its toxicity.
Conceptual Problems
Q10.3.1
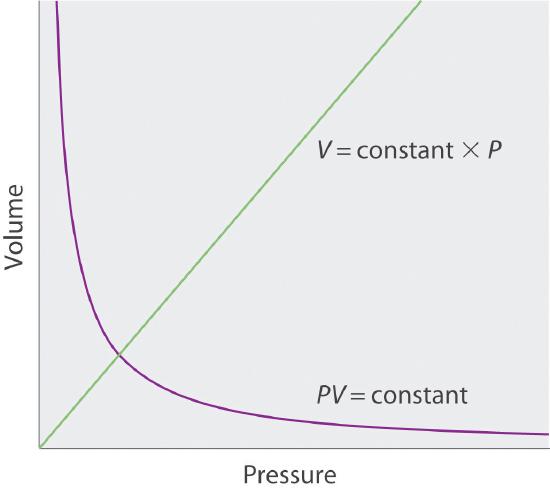
Sketch a graph of the volume of a gas versus the pressure on the gas. What would the graph of V versus P look like if volume was directly proportional to pressure?
Q10.3.2
What properties of a gas are described by Boyle’s law, Charles’s law, and Avogadro’s law? In each law, what quantities are held constant? Why does the constant in Boyle’s law depend on the amount of gas used and the temperature at which the experiments are carried out?
Q10.3.3
Use Charles’s law to explain why cooler air sinks.
Q10.3.4
Use Boyle’s law to explain why it is dangerous to heat even a small quantity of water in a sealed container.
Numerical Problems
Q10.3.5
A 1.00 mol sample of gas at 25°C and 1.0 atm has an initial volume of 22.4 L. Calculate the results of each change, assuming all the other conditions remain constant.
- The pressure is changed to 85.7 mmHg. How many milliliters does the gas occupy?
- The volume is reduced to 275 mL. What is the pressure in millimeters of mercury?
- The pressure is increased to 25.3 atm. What is the temperature in degrees Celsius?
- The sample is heated to 30°C. What is the volume in liters?
- The sample is compressed to 1255 mL, and the pressure is increased to 2555 torr. What is the temperature of the gas in kelvins?
S10.3.5
- 1.99 × 105 mL
- 6.19 × 104 mmHg
- 7270°C
- 22.8 L
- 51.4 K
Q10.3.6
A 1.00 mol sample of gas is at 300 K and 4.11 atm. What is the volume of the gas under these conditions? The sample is compressed to 6.0 atm at constant temperature, giving a volume of 3.99 L. Is this result consistent with Boyle’s law?
Conceptual Problems
Q10.5.1
Why are so many industrially important reactions carried out in the gas phase?
Q10.5.2
The volume of gas produced during a chemical reaction can be measured by collecting the gas in an inverted container filled with water. The gas forces water out of the container, and the volume of liquid displaced is a measure of the volume of gas. What additional information must be considered to determine the number of moles of gas produced? The volume of some gases cannot be measured using this method. What property of a gas precludes the use of this method?
Q10.5.3
Equal masses of two solid compounds (A and B) are placed in separate sealed flasks filled with air at 1 atm and heated to 50°C for 10 hours. After cooling to room temperature, the pressure in the flask containing A was 1.5 atm. In contrast, the pressure in the flask containing B was 0.87 atm. Suggest an explanation for these observations. Would the masses of samples A and B still be equal after the experiment? Why or why not?
Numerical Problems
Q10.5.4
Balance each chemical equation and then determine the volume of the indicated reactant at STP that is required for complete reaction. Assuming complete reaction, what is the volume of the products?
- SO2(g) + O2(g) → SO3(g) given 2.4 mol of O2
- H2(g) + Cl2(g) → HCl(g) given 0.78 g of H2
- C2H6(g) + O2(g) → CO2(g) + H2O(g) given 1.91 mol of O2
Q10.5.5
During the smelting of iron, carbon reacts with oxygen to produce carbon monoxide, which then reacts with iron(III) oxide to produce iron metal and carbon dioxide. If 1.82 L of CO2 at STP is produced,
- what mass of CO is consumed?
- what volume of CO at STP is consumed?
- how much O2 (in liters) at STP is used?
- what mass of carbon is consumed?
- how much iron metal (in grams) is produced?
Q10.5.6
Complete decomposition of a sample of potassium chlorate produced 1.34 g of potassium chloride and oxygen gas.
- What is the mass of KClO3 in the original sample?
- What mass of oxygen is produced?
- What is the volume of oxygen produced at STP?
S10.5.6
- 2.20 g KClO3
- 0.863 g O2
- 604 mL O2
Q10.5.7
The combustion of a 100.0 mg sample of an herbicide in excess oxygen produced 83.16 mL of CO2 and 72.9 mL of H2O vapor at STP. A separate analysis showed that the sample contained 16.44 mg of chlorine. If the sample is known to contain only C, H, Cl, and N, determine the percent composition and the empirical formula of the herbicide.
Q10.5.8
The combustion of a 300.0 mg sample of an antidepressant in excess oxygen produced 326 mL of CO2 and 164 mL of H2O vapor at STP. A separate analysis showed that the sample contained 23.28% oxygen. If the sample is known to contain only C, H, O, and N, determine the percent composition and the empirical formula of the antidepressant.
S10.5.8
Percent composition: 58.3% C, 4.93% H, 23.28% O, and 13.5% N; empirical formula: C10H10O3N2
Conceptual Problems
Q10.8.1
Which of the following processes represents effusion, and which represents diffusion?
- helium escaping from a hole in a balloon
- vapor escaping from the surface of a liquid
- gas escaping through a membrane
Q10.8.2
Which postulate of the kinetic molecular theory of gases most readily explains the observation that a helium-filled balloon is round?
Q10.8.3
Why is it relatively easy to compress a gas? How does the compressibility of a gas compare with that of a liquid? A solid? Why? Which of the postulates of the kinetic molecular theory of gases most readily explains these observations?
Q10.8.4
What happens to the average kinetic energy of a gas if the rms speed of its particles increases by a factor of 2? How is the rms speed different from the average speed?
Q10.8.5
Which gas—radon or helium—has a higher average kinetic energy at 100°C? Which has a higher average speed? Why? Which postulate of the kinetic molecular theory of gases most readily supports your answer?
Q10.8.6
What is the relationship between the average speed of a gas particle and the temperature of the gas? What happens to the distribution of molecular speeds if the temperature of a gas is increased? Decreased?
Q10.8.7
Qualitatively explain the relationship between the number of collisions of gas particles with the walls of a container and the pressure of a gas. How does increasing the temperature affect the number of collisions?
Q10.8.8
What happens to the average kinetic energy of a gas at constant temperature if the
- volume of the gas is increased?
- pressure of the gas is increased?
Q10.8.9
What happens to the density of a gas at constant temperature if the
- volume of the gas is increased?
- pressure of the gas is increased?
Q10.8.10
Use the kinetic molecular theory of gases to describe how a decrease in volume produces an increase in pressure at constant temperature. Similarly, explain how a decrease in temperature leads to a decrease in volume at constant pressure.
Q10.8.11
Graham’s law is valid only if the two gases are at the same temperature. Why?
Q10.8.12
If we lived in a helium atmosphere rather than in air, would we detect odors more or less rapidly than we do now? Explain your reasoning. Would we detect odors more or less rapidly at sea level or at high altitude? Why?
Numerical Problems
Q10.8.13
At a given temperature, what is the ratio of the rms speed of the atoms of Ar gas to the rms speed of molecules of H2 gas?
S10.8.13
At any temperature, the rms speed of hydrogen is 4.45 times that of argon.
Q10.8.14
At a given temperature, what is the ratio of the rms speed of molecules of CO gas to the rms speed of molecules of H2S gas?
Q10.8.15
What is the ratio of the rms speeds of argon and oxygen at any temperature? Which diffuses more rapidly?
Q10.8.16
What is the ratio of the rms speeds of Kr and NO at any temperature? Which diffuses more rapidly?
Q10.8.17
Deuterium (D) and tritium (T) are heavy isotopes of hydrogen. Tritium has an atomic mass of 3.016 amu and has a natural abundance of 0.000138%. The effusion of hydrogen gas (containing a mixture of H2, HD, and HT molecules) through a porous membrane can be used to obtain samples of hydrogen that are enriched in tritium. How many membrane passes are necessary to give a sample of hydrogen gas in which 1% of the hydrogen molecules are HT?
Q10.8.18
Samples of HBr gas and NH3 gas are placed at opposite ends of a 1 m tube. If the two gases are allowed to diffuse through the tube toward one another, at what distance from each end of the tube will the gases meet and form solid NH4Br?
Conceptual Problems
Q10.9.1
What factors cause deviations from ideal gas behavior? Use a sketch to explain your answer based on interactions at the molecular level.
Q10.9.2
Explain the effect of nonzero atomic volume on the ideal gas law at high pressure. Draw a typical graph of volume versus 1/P for an ideal gas and a real gas.
Q10.9.3
For an ideal gas, the product of pressure and volume should be constant, regardless of the pressure. Experimental data for methane, however, show that the value of PV decreases significantly over the pressure range 0 to 120 atm at 0°C. The decrease in PV over the same pressure range is much smaller at 100°C. Explain why PV decreases with increasing temperature. Why is the decrease less significant at higher temperatures.
Q10.9.4
What is the effect of intermolecular forces on the liquefaction of a gas? At constant pressure and volume, does it become easier or harder to liquefy a gas as its temperature increases? Explain your reasoning. What is the effect of increasing the pressure on the liquefaction temperature?
Q10.9.5
Describe qualitatively what a and b, the two empirical constants in the van der Waals equation, represent.
Q10.9.6
In the van der Waals equation, why is the term that corrects for volume negative and the term that corrects for pressure positive? Why is n/V squared?
Q10.9.7
Liquefaction of a gas depends strongly on two factors. What are they? As temperature is decreased, which gas will liquefy first—ammonia, methane, or carbon monoxide? Why?
Q10.9.8
What is a cryogenic liquid? Describe three uses of cryogenic liquids.
Q10.9.9
Air consists primarily of O2, N2, Ar, Ne, Kr, and Xe. Use the concepts discussed in this chapter to propose two methods by which air can be separated into its components. Which component of air will be isolated first?
Q10.9.10
How can gas liquefaction facilitate the storage and transport of fossil fuels? What are the potential drawbacks to these methods?
Numerical Problems
Q10.9.11
The van der Waals constants for xenon are a = 4.19 (L2·atm)/mol2 and b = 0.0510 L/mol. If a 0.250 mol sample of xenon in a container with a volume of 3.65 L is cooled to −90°C, what is the pressure of the sample assuming ideal gas behavior? What would be the actual pressure under these conditions?
Q10.9.12
The van der Waals constants for water vapor are a = 5.46 (L2·atm)/mol2 and b = 0.0305 L/mol. If a 20.0 g sample of water in a container with a volume of 5.0 L is heated to 120°C, what is the pressure of the sample assuming ideal gas behavior? What would be the actual pressure under these conditions?