3.8 Gas Evolution Reactions
- Page ID
- 164013
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify common compounds that react with acids/bases to produce gases
- Write balanced equations to represent gas evolution reactions
Gas Evolving Reactions
A gas evolution reaction is a chemical process that produces a gas, such as oxygen or carbon dioxide. In the following examples, an acid reacts with a carbonate, producing salt, carbon dioxide, and water, respectively. For example, nitric acid reacts with sodium carbonate to form sodium nitrate, carbon dioxide, and water (Table \(\PageIndex{1}\)):
\[\ce{2HNO3(aq)+Na2CO3(aq)→2NaNO3(aq)+CO2(g)+H2O(l)}\]
Sulfuric acid reacts with calcium carbonate to form calcium sulfate, carbon dioxide, and water:\[\ce{H2SO4(aq) + CaCO3(aq) → CaSO4(aq) + CO2(g)+H2O(l)}\]Hydrochloric acid reacts with calcium carbonate to form calcium chloride, carbon dioxide, and water:\[\ce{2HCl(aq) + CaCO3(aq) → CaCl2(aq) + CO2(g) + H2O(l)}\]Figure \(\PageIndex{1}\) demonstrates this type of reaction:
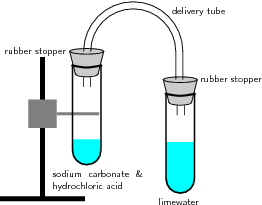
Figure \(\PageIndex{1}\): Reaction of acids with carbonates. In this reaction setup, lime water is poured into one of the test tubes and sealed with a stopper. A small amount of hydrochloric acid is carefully poured into the remaining test tube. A small amount of sodium carbonate is added to the acid, and the tube is sealed with a rubber stopper. The two tubes are connected. As a result of the acid-carbonate reaction, carbon dioxide is produced and the lime water turns milky.
The test tube on the right contains limewater (a solution of calcium hydroxide, \(Ca(OH)_2\)). On the left, a solution of hydrochloric acid has been added to a solution of sodium carbonate to generate \(CO_2(g)\). The test tubes are sealed with rubber stoppers and connected with a delivery tube. As the reaction proceeds, the limewater on the right turns from clear to milky; this is due to the \(CO_2(g)\) reacting with the aqueous calcium hydroxide to form calcium carbonate, which is only slightly soluble in water. The entire experiment is illustrated in the following video:
Video \(\PageIndex{1}\): Carbon Dioxide (\(CO_2\)) & Limewater (Chemical Reaction). Carbon dioxide (formed from bicarbonate) and an acid react with limewater, turning it from clear to milky.
When this experiment is repeated with nitric or sulfuric acid instead of \(HCl\), it yields the same results: the clear limewater turns milky, indicating the production of carbon dioxide. Another method to chemically generate gas is the oxidation of metals in acidic solutions. This reaction will yield a metal salt and hydrogen gas.
\[\ce{2HCl (aq) + Zn(s) \rightarrow ZnCl_2 (aq) + H_2 (g)}\]
Here, hydrochloric acid oxidizes zinc to produce an aqueous metal salt and hydrogen gas bubbles. Recall that oxidation refers to a loss of electrons, and reduction refers to the gain of electrons. In the above redox reaction, neutral zinc is oxidized to \(Zn^{2+}\), and the acid, \(H^+\), is reduced to \(H_2(g)\). The oxidation of metals by strong acids is another common example of a gas evolution reaction.
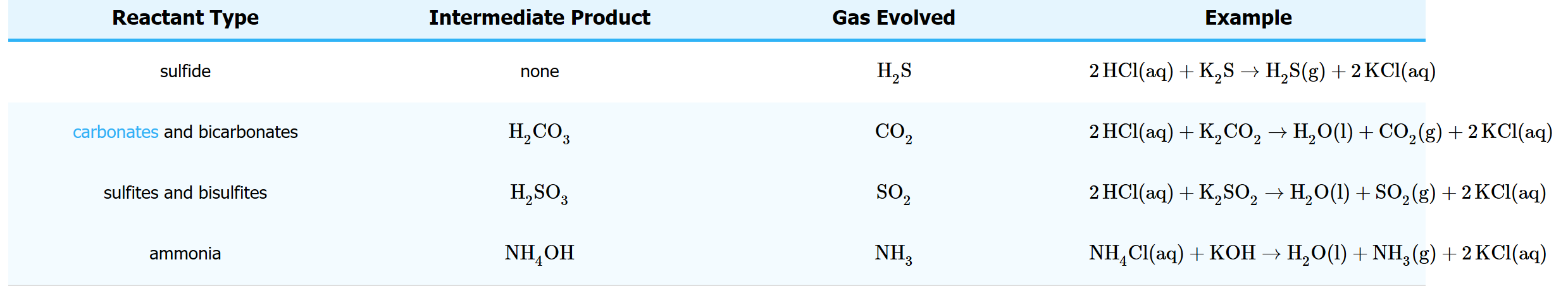
Table 1: Common gas forming reactions.
Exercise \(\PageIndex{1}\)
Adding vinegar(active ingredient is acetic acid) to milk makes "buttermilk". The "buttermilk" is then mixed added to a pancake mix that contains sodium bicarbonate(baking soda) to make pancakes "fluffy". Write a balanced chemical reaction showing how acetic acid reacts with sodium bicarbonate. Why do think it is used to make pancakes fluffy?
- Answer
-
NaHCO3(s) + HC2H3O2(aq) → NaC2H3O2(aq) + H2CO3
NaHCO3(s) + HC2H3O2(aq) → NaC2H3O2(aq) + H2O(l) + CO2(g)
H2CO3 is an intermediate product that breaks down into water and carbon dioxide gas. The carbon dioxide "bubbles" is what contributes to the "fluffiness" of the pancakes.
Note: that is why you mix the dry ingredients and the wet ingredients separately before you combine them. If you over mix or leave your batter for too long, the carbon dioxide will escape in the air and your pancakes will be flat. :(
Contributors:
- Original Article: 7.8: Acid–Base and Gas Evolution Reactions
- Modified by Ronia Kattoum (UA of Little Rock)

