6: The Structure of Atoms
- Page ID
- 168600
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Speed of Light
Exercise \(\PageIndex{1}\)
A device emits light at 512.8 nm. What is the frequency of this radiation?
- Answer
-
5.851 x 1014 Hz
\[v = \frac{c}{\lambda }\]
\[v\;=\;(\frac{2.99\;*\;10^{8}\;ms^{-1} }{512.8\;*\;10^{-9}})\]
\[v\; = 5.8307\;*\;10^{14}\;Hz\]
Exercise \(\PageIndex{2}\)
What is the wavelength of a photon having a frequency of 75.9 THz? (1 THz = 1015 Hz)
- Answer
-
3.94 nm
Exercise \(\PageIndex{3}\)
What is the frequency of gamma ray radiation that has a wavelength of 15.6 pm?
- Answer
-
1.92 × 1019 s–1
Exercise \(\PageIndex{4}\)
If a cordless phone operates at a frequency of 6.23 × 108 s–1, what is the wavelength of this radiation?
- Answer
-
0.480 m
Exercise \(\PageIndex{5}\)
A device operates at a frequency of 1.25 x 1014 Hz. What is the wavelength of this radiation?
- Answer
-
0.239 nm
Radiation and Planck's Constant
Exercise \(\PageIndex{1}\)
What is the energy of a photon of electromagnetic radiation with a wavelength of 256.8 nm?
- Answer
-
7.714 x 10-19 J
\[E=\frac{h*c}{\lambda }\]
\[E=\frac{(6.625*10^{-34}\;J*s)*(2.99*10^{8}\;m/s)}{(256.8*10^{-9}\;m)}=7.7136*10^{-19}\;J\]
Exercise \(\PageIndex{2}\)
What is the wavelength of a photon that has an energy of 3.861 x 104 J
- Answer
-
5.130 x 10-21 nm
Exercise \(\PageIndex{3}\)
What is the energy of a photon of electromagnetic radiation with a frequency of 9.48 x 1014 Hz?
- Answer
-
6.28 x 10-19 J
Per Photon or Per Mole
Exercise \(\PageIndex{4}\)
A red laser pointer emits light at a wavelength of 580.9 nm. If the laser emits 1.84 × 10–4 J of energy per second in the form of visible radiation, how many photons per second are emitted from the laser?
- Answer
-
5.40 × 1014 photons/sec
\[E=\frac{hc}{\lambda }\]
\[E=\frac{(6.625*10^{-34}\;J/s)(2.99*10^{8}\;m/s)}{(580.9*10^{-9})\;m}=5.395*10^{14}\;photons/sec\]
Exercise \(\PageIndex{5}\)
What is the energy per mole of photons of light with a wavelength of 690.8 nm?
- Answer
-
8.210 × 102 kJ/mol
\[E_{per\;mole}=\frac{N_{a}hc}{\lambda }\]
\[E_{per\;mole}=\frac{(6.022*10^{23})(6.625*10^{-34})(2.99*0^{8})}{145.3*10^{-9}}=820979\;J/mol\]
Exercise \(\PageIndex{6}\)
What is the energy per mole of photons of light with a frequency of 2.98 × 1015 Hz?
- Answer
-
1.19 × 103 kJ/mol
Exercise \(\PageIndex{7}\)
If the energy of 1.00 mole of photons is 658 kJ, what is the wavelength of the light?
- Answer
-
181 nm
Exercise \(\PageIndex{8}\)
A light emitting diode (L.E.D.) emits photons with an energy of 6.359 x 10-19 J. What is the energy per mole of photons emitted?
- Answer
-
3.829 x 105 J/mol
Exercise \(\PageIndex{9}\)
What is the binding energy of an electron in a photosensitive metal (in kJ/mol) if the longest wavelength of light that can eject electrons from the metal is 459.0 nm?
- Answer
-
259.9 kJ/mol
Exercise \(\PageIndex{10}\)
The energy required to break one mole of fluorine-fluorine bonds in F2 is 155 kJ/mol. What is the longest wavelength of light capable of breaking a single F-F bond?
- Answer
-
770 nm
Rydberg Equation
Exercise \(\PageIndex{1}\)
What is the wavelength of light emitted when an electron in a hydrogen atom undergoes a transition from energy level n = 3 to level n = 1? (c = 3.00 × 108 m/s, h = 6.63 × 10–34 J·s, RH = 2.179 × 10–18 J)
- Answer
-
102 nm
\[\frac{hc}{\lambda } = R_{H}\left [(\frac{1}{(n_{1})^{2}})-(\frac{1}{(n_{2})^{2}}) \right ]\]
\[\frac{(6.63*10^{-34})(3.00*10^{8})}{\lambda }=(2.179*10^{-18})(\frac{1}{1}-\frac{1}{9})\]
\[\lambda = \frac{1.981*10^{-25}}{1.937*10^{-18}}\]\[\lambda = 1.023*10^{-7}\;m\]
Exercise \(\PageIndex{2}\)
The electron in a hydrogen atom, originally in level n = 5, undergoes a transition to a lower level by emitting a photon of wavelength 1583 nm. What is the final level of the electron? (c = 3.00 × 108 m/s, h = 6.626 × 10–34 J·s, RH = 2.179 × 10–18 J)
- Answer
-
3
Emission Lines
Exercise \(\PageIndex{3}\)
If a hydrogen atom in the excited n = 5 state relaxes to the ground state, what is the maximum number of possible emission lines?
- Answer
-
15
de Broglie Wavelength
Exercise \(\PageIndex{1}\)
For a proton (mass = 1.673 × 10–27 kg) moving with a velocity of 8.63 × 104 m/s, what is the de Broglie wavelength of the proton (in pm)?
- Answer
-
4.59 pm
\[E = \frac{1}{2}mv^{2}\]
\[E = \frac{1}{2}(1.673*10^{-27})(8.63*10^{4})^{2} = 6.229*10^{-18}\;J\]
\[\lambda = \frac{h}{\sqrt{2Em}}\]
\[\lambda = \frac{6.626*10^{-34}}{\sqrt{2*(6.229*10^{-18})*(1.673*10^{-27})}}\]
\[\lambda = 4.588*10^{-12}\;m=4.588\;pm\]
Exercise \(\PageIndex{1}\)
What is the de Broglie wavelength of an electron traveling at 15.9% of the speed of light? (c = 3.00 × 108 m/s, h = 6.63 × 10–34 J·s, me = 9.109 × 10–31 J)
- Answer
-
1.52 × 10–11 m
Exercise \(\PageIndex{1}\)
If the de Broglie wavelength of an electron is 90.2 nm, what is its velocity? (The mass of an electron is 9.11 × 10–31 kg.)
- Answer
-
8.06 × 103 m/s
Exercise \(\PageIndex{1}\)
What is the de Broglie wavelength of a 250-g baseball traveling at 85 mph? (1 mi = 1.609 km and h = 6.63 × 10–34 J·s)
- Answer
-
7.0 × 10-35 m
Quantum Numbers
Exercise \(\PageIndex{1}\)
What is the value of the orbital angular momentum quantum number (l) for an electron in a 4f orbital?
a. 1 b. 4 c. 2 d. 3 e. 0
- Answer
-
d. 3
Exercise \(\PageIndex{2}\)
How many orbitals have the following set of quantum numbers: n = 5, l= 3, ml= –1?
a. 0 b. 1 c. 3 d. 6 e. 7
- Answer
-
b. 1 orbital
Exercise \(\PageIndex{3}\)
Which of the following sets of quantum numbers is not allowed?
- n = 4, l = 2, ml = -2
- n = 6, l = 1, ml = 1
- n = 5, l = 4, ml = -4
- n = 2, l = 1, ml = -3
- n = 6, l = 3, ml = -2
- Answer
-
d. n = 2, l = 1, ml = -3
Exercise \(\PageIndex{4}\)
What is the total number of orbitals having n = 3 and l= 2?
- 1 orbital
- 3 orbitals
- 5 orbitals
- 7 orbitals
- 10 orbitals
- Answer
-
a. 1 orbital
Exercise \(\PageIndex{5}\)
How many f orbitals are in the n = 4 shell?
- 5 f orbitals
- 8 f orbitals
- 3 f orbitals
- 1 f orbital
- 7 f orbitals
- Answer
-
e. 7 f orbitals
Exercise \(\PageIndex{6}\)
What type of orbital is designated n = 4, l= 2, ml= 2?
a. 4f b. 4d c. 4p d. 4g e. 4s
- Answer
-
b. 4d
Exercise \(\PageIndex{7}\)
Which type of orbital is designated n = 2 and l= 1?
a. 2p b. 3s c. 4d d. 1f e. 2d
- Answer
-
a. 2p
Exercise \(\PageIndex{8}\)
Which of the following orbital can be represented by n = 4, l= 3, and ml = –2?
a. 4s b. 4p c. 4d d. 4f e. None of these
- Answer
-
d. 4f
Exercise \(\PageIndex{9}\)
A possible value of the magnetic quantum number ml for a 5p electron is
a. 1 b. 4 c. 5 d. -6 e. 3
- Answer
-
a. 1
Exercise \(\PageIndex{10}\)
How many values are there for the magnetic quantum number (ml) when the value of the angular momentum quantum number (l) is 4?
a. 11 b. 9 c. 2 d. 4 e. 15
- Answer
-
b. 9
Exercise \(\PageIndex{11}\)
What is the total number of subshells found in the n = 7 shell?
a. 7 b. 49 c. 6 d. 8 e. 9
- Answer
-
a. 7 subshells
Exercise \(\PageIndex{12}\)
Which of the following sets of quantum numbers refers to a 4d orbital?
- n = 2, l = 1, ml = -1
- n = 2, l = 4, ml = -1
- n = 4, l = 2, ml = -1
- n = 4, l = 3, ml = 0
- n = 4, l = 3, ml = +2
- Answer
-
c. n = 4, l = 2, ml = -1
Exercise \(\PageIndex{13}\)
The (principal quantum number) n = ____ shell is the lowest that may contain s-orbitals.
a. 1 b. 2 c. 3 d. 4 e. 5
- Answer
-
a. 1
Orbitals
Exercise \(\PageIndex{1}\)
A 4d orbital has ?
- 3 planar nodes and 3 spherical nodes
- 1 planar node and 1 spherical nodes
- 2 planar nodes and 1 spherical node
- 2 planar nodes and 4 spherical nodes
- 4 planar nodes and 2 spherical nodes
- Answer
-
c. 2 planar nodes and 1 spherical node
Exercise \(\PageIndex{2}\)
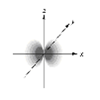
Which of the following is a representation of a 3dxy orbital?
- Answer
-
a.
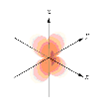
Exercise \(\PageIndex{3}\)
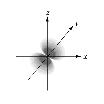
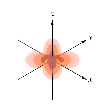
Which of the following orbital boundary surfaces represent d-orbitals?
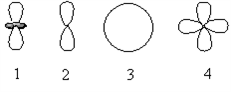
a. 1 only b. 3 only c. 2 only d. 1 and 2 e. 1 and 4
- Answer
-
e. 1 and 4
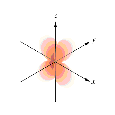
Exercise \(\PageIndex{4}\)
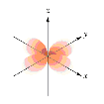
Which of the following orbital boundary surfaces is a representation of a 3dz2 orbital?
- Answer
-
a.
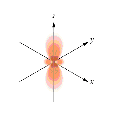
Paramagnetic, Diamagnetic and Ferromagnetic
Exercise \(\PageIndex{1}\)
Which of the following statements is/are CORRECT?
- A paramagnetic substance is attracted to a magnetic field.
- An atom with no unpaired electrons is ferromagnetic.
- Atoms with one or more unpaired electrons are paramagnetic.
a. 1 only b. 3 only c. 3 only d. 1 and 3 e. 1,2, and 3
- Answer
-
d. 1 and 3
Exercise \(\PageIndex{2}\)
Which of the following statements is/are CORRECT?
- A diamagnetic substance is strongly attracted to a magnetic field.
- Substances that retain their magnetism after they are withdrawn from a magnetic field are called ferromagnetic.
- Most transition metals and all lanthanide metals are ferromagnetic.
a. 1 only b. 2 only c. 3 only d. 1 and 3 e. 1,2, and 3
- Answer
-
b. 2 only
Quantum Numbers and Spin
Exercise \(\PageIndex{3}\)
What is the value of the spin quantum number for an electron in a 3d orbital?
- 3
- 2
- either \(+\frac{1}{2}\) or \(-\frac{1}{2}\)
- \(-\frac{1}{2}\)
- \(+\frac{1}{2}\)
- Answer
-
c. either \(+\frac{1}{2}\) or \(-\frac{1}{2}\)
Exercise \(\PageIndex{4}\)
Which of the following sets of quantum numbers (n, l, ml, ms) is not permissible?
- 2, 2, 1, \(+\frac{1}{2}\)
- 3, 1, 0, \(-\frac{1}{2}\)
- 1, 0, 0, \(+\frac{1}{2}\)
- 2, 1, 0, \(+\frac{1}{2}\)
- 4, 0, 0, \(-\frac{1}{2}\)
- Answer
-
a. 2, 2, 1, \(+\frac{1}{2}\)