4.4: Polyatomic Ions
- Page ID
- 280727
Polyatomic Ions
Polyatomic Ions are charged molecules, where the total number of protons in all bonded atoms is \(\neq\) to the total number of electrons in all bonded electrons. When writing structural formulas for ions we place the formula in brackets and the charge as a superscript outside of the bracket. The number of protons is the total number of protons in all atoms, while the number of protons minus the number of electrons is the charge of the atom. That is the charge of the ion is the charge of all protons [+ value] plus the charge of all electrons [- value].
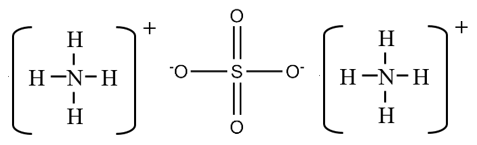
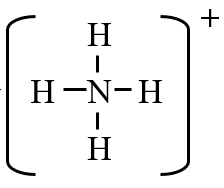
- Ammonium is a polyatomic cation, NH4+.
Exercise \(\PageIndex{1}\)
How many protons and electrons are in ammonia
- Answer
-
There are 7 protons from the nitrogen and 4 from the four hydrogen (1 each) and so a total of 11 protons are in this polyatomic ion, which as drawn, has 4 covalent bonds. Since the charge is [+1], \[\#protons -\#electrons= charge \\ \#electrons=\#protons- charge\\ \#electrons=11-1=10 \nonumber \]
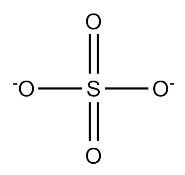
- Sulfate is a polyatomic anion, SO4-2.
Exercise \(\PageIndex{1}\)
How many protons and electrons are in sulfate?
- Answer
-
There are 16 protons from the sulfur and 32 from the four oxygen (8 each) and so a total of 48 protons are in this polyatomic ion, which as drawn, has 6 covalent bonds (note two are double bonds). Since the charge is [-2], \[\#protons -\#electrons= charge \\ \#electrons=\#protons- charge\\ \#electrons=48-(-2)=50 \nonumber\]
Formula of Polyatomic Ions
The rules for formula of polyatoic ions are the same as for monatomic ions. The formula of the neutral compound between ammonium and sulfate is (NH4)2SO4.