3.5: The Periodic Table
- Page ID
- 278751
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Learning objectives
- Identify the groups of the periodic table
- Identify elements as metals, nonmetals, metalloids
- Infer likely properties of elements based on their position on the periodic table.
Classification of the Periodic Table
If you tried to click on any of the above elements, the details of each element may seem overwhelming and it may seem incredibly difficult to find patterns. However, the beauty of the periodic table is found in it's periodicity, repeating patterns that help us predict physical and chemical properties of various elements. Thus the periodic table can also be split into seven rows, known as periods (Figure \(\PageIndex{4}\)). At first glance, it may seem like the two lone rows at the bottom of the period table, known as the actinide and lanthanide series should be periods 8 and 9. However, if you look carefully, these two row should actually be wedged in the 6th and 7th period, respectfully, in order to follow the order of increasing atomic number. Those rows are kept at the bottom to decrease the width of the periodic table and are also known as the inner transition metals.
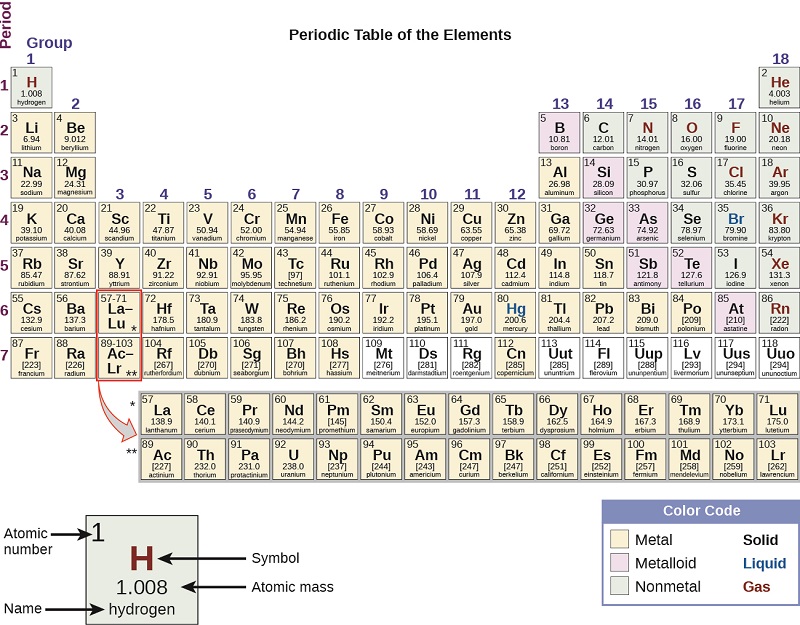
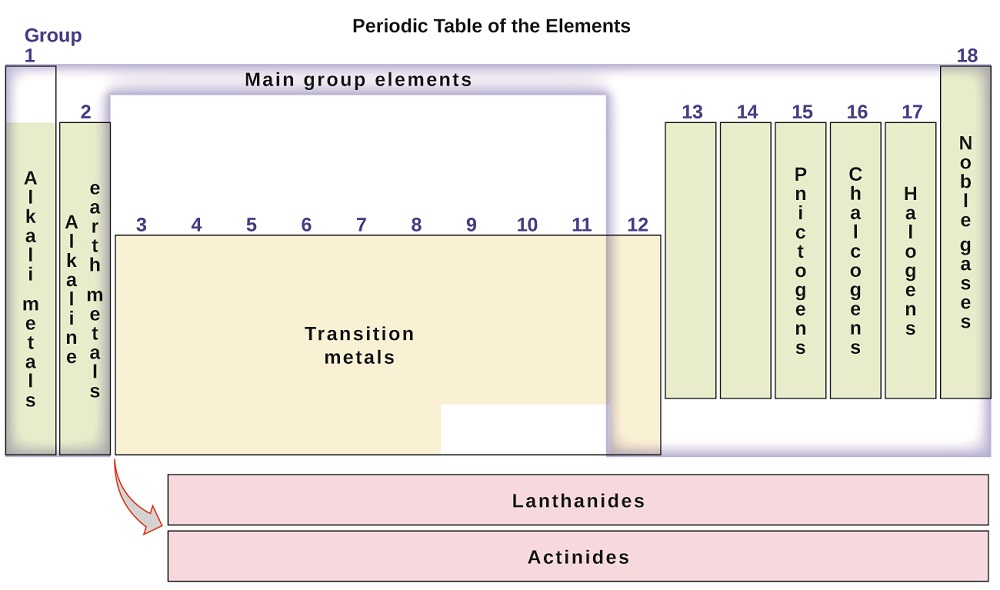
Groups can be numbered as 1-18 (Figures \(\PageIndex{1}\) or \(\PageIndex{2}\)) or they can be numbered 1-8 followed by "A" or "B" (Figure \(\PageIndex{3}\)). The "A" designation represents the main group elements (Columns 1-2, and Columns 13-18) The "B" designation represents transition elements (Columns 3-12). Either group numbering system is acceptable, but we will see later that there is a benefit for knowing the group numbers with the "A" and "B" designation. In addition, some of the individual groups have a particular group name. For example, Group 17 or 7A elements are also known as the halogens. Group 2 or 2A elements are known as the alkaline group metals. Group names are commonly used by chemists and are worth knowing. But, most importantly, elements in the same groups tend to have similar chemical and physical properties. We will also discuss the reason behind such patterns later in this text. Figure \(\PageIndex{2}\) provides the common names of the groups.
Exercise \(\PageIndex{1}\)
Which of the following elements will most likely have similar chemical properties to fluorine?
A) neon
B) nitrogen
C) iodine
D) sulfur
- Answer
-
C) iodine. Elements in the same group have similar chemical properties, not the same periods.
The Families of the Periodic table
Exercise \(\PageIndex{2}\)
What alkali metal is found in period 4?
A) carbon
B) potassium
C) calcium
D) Titanium
- Answer
-
B) Potassium is in Group (Column) 1A and is in the 4th period (row).
Figure \(\PageIndex{3}\): Modern Family or group numbering system for the Periodic Table along with the names of the families.
Exercise \(\PageIndex{3}\)
What is the group number for oxygen?
A) 16
B) 6
C) 16A
D) Both A and B
- Answer
If you start at boron and draw "stairs" down to astatine, the seven elements that are "on" are the metalloids or semi-metals (Figures \(\PageIndex{3}\)). Everything to the left of the "stairs" with the exception of hydrogen is a metal. Everything to the right of the "stairs", in addition to hydrogen, is a nonmetal. This is essential for describing common physical properties for each of the three categories and will be used in nomenclature of chemical compounds.
Exercise \(\PageIndex{4}\)
What is true regarding the classification of the following silicon? (select all that apply)
A) group 4A
B) metalloid
C) main group element
D) period 14
E) nonmetal
- Answer
-
A, B, and C are all correct. D is incorrect. The period (row) is 3. The group is 13. E is incorrect because silicon sits on the stairs and is one of the metalloids.
Physical Properties of Metals:
Note, these can be related to the fact that electrons in metallic bonds are loosely bonded between multiple core atoms and freely flow from one atom to another.
- Conduction (heat and electricity)
- Malleability (can be hammered into thin sheets)
- Ductility (can be pulled into wires)
- Lustrous (Shiny appearance)
Physical Properties of Non-Metals:
Note, nonmetals are held together by directional covalent bonds of shared electrons, and this relates to their properties.
- Poor Conductors
- Nonlusterous
- Nonmalleable
- Brittle
- Brightly colored
They have both properties of metals and non-metals. One of the most important physical properties of metalloids is their semi-conductive properties.
Natural States of Atoms
If you go to the Pubchem periodic table and click "standard state, you will see 2 elements are liquid, 11 are gasses, and one (Og) is "expected" to be a gas. If you click on Og and go to it's PubChem elemental page you see the most stable isotope has a half life of 0.89 millliseconds, and only a few atoms have ever been produced. You are required to know these, and note, several elements like Ga, Cs and Fr melt at very low temperatures and so may actually be liquids at room temperature
Liquids: Hg, Br2, ( Ga, mp=85.58oF; Cs, mp=83.3oF; Fr, mp=80.6oF and Rb, mp=103.1 oF)
Gasses: Noble Gasses and Lighter diatomics, H2, N2, O2, F2, & Cl2
Solids: All other elements
Vocabulary
Contributors and Attributions
Robert E. Belford (University of Arkansas Little Rock; Department of Chemistry). The breadth, depth and veracity of this work is the responsibility of Robert E. Belford, rebelford@ualr.edu. You should contact him if you have any concerns. This material has both original contributions, and content built upon prior contributions of the LibreTexts Community and other resources, including but not limited to:
- Anonymous
- Modifications by Ronia Kattoum
- Elena Lisitsyna (H5P interactive modules)

