5.8: Dehydration of Aldol Products - Synthesis of Enones
- Page ID
- 469389
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Aldol Condensation
Reactions in which a larger molecule is formed from smaller components, with the elimination of a very small by-product such as water are termed Condensations. Hence the following examples are properly referred to as aldol condensations.
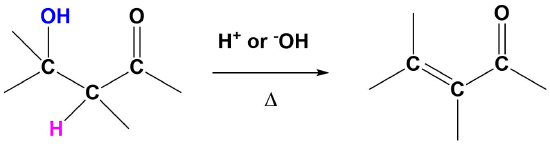
Dehydration of Aldol Products to Synthesize α, β Unsaturated carbonyl (enones)
The products of aldol reactions, with heating, often undergo a subsequent elimination of water, made up from an alpha-hydrogen and the beta-hydroxyl group. The product of this acid or base-catalyzed E1cB elimination reaction (Section 11-10) reaction is an α,β-unsaturated aldehyde or ketone (Enones). Although there may be multiple position where the alkene may form, it will always prefer to be in conjugation with the carbonyl.
Conjugated enone products are more stable than non-conjugated due to extended P orbital overlap. Conjugation of the p electrons of the alkene and carbonyl bonds provide a molecular-orbital description showing the interaction of p electrons of all four atoms. The additional stability provided by the conjugated carbonyl system of the product makes many aldol reactions thermodynamically factorable.
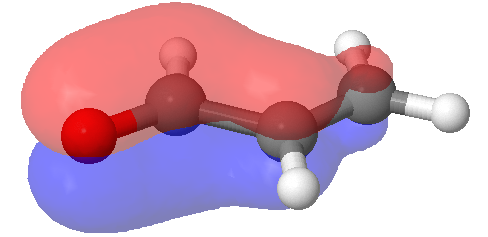
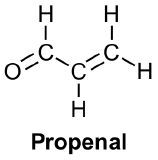
A representation of pi bonding molecular orbitals of the conjugated enone, propenal, are delocalized through p-orbital overlap
The elimination of water from the reaction mixture can be used to drive the equilibrium towards the products by Le Chatelier’s principle. This coupled with the thermodynamic stability of the conjugated product allow for good reaction yields when the formation of the initial aldol intermediate is unfavorable (ketones & sterically hindered aldehydes).
Predicting the Product of an Enone Formation
Stereochemical Considerations
When aldehyde starting materials are used for an aldol condensation, there is the possibility of forming both E and Z alkene isomers. When symmetrical ketones are used, the alkene formed lacks the ability to form isomers so a single product is made.
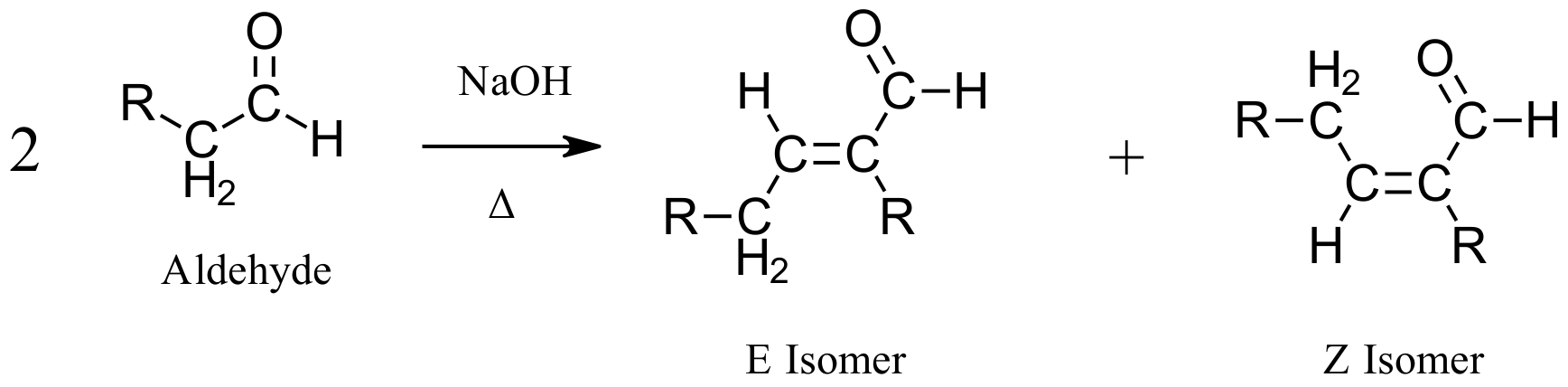
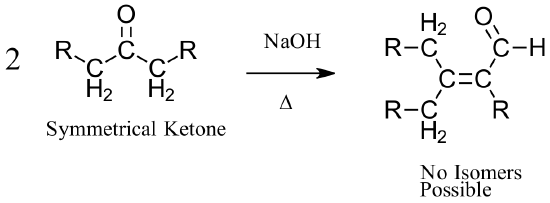
Examples
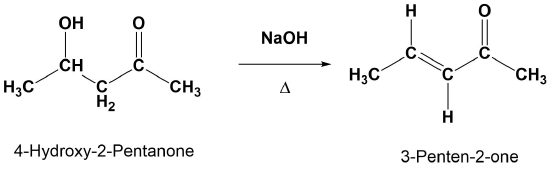
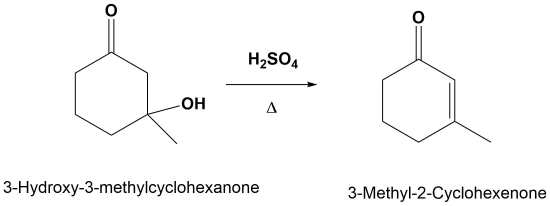
Mechanism
Base Catalyzed Mechanism
1) Form an enolate
The mechanism starts with the base removing an alpha-hydrogen to form an enolate ion.
2) Form the enone
The alkoxide reforming the carbonyl C=O bond promotes the elimination of alcohol OH as a leaving group which reforms the base catalyst. Although the base catalyzed elimination of alcohols is rare, it happens in this case in part due to the stability of the conjugated enone product. This is an example of an E1CB reaction.
Acidic Conditions Mechanism
1) Protonation
The mechanism starts with the two step tautomerization process to form an enol.
2) Form an enol
3) Protonation
Protonation of the alcohol OH increases its ability to act as a leaving group.
4) Elimination
Lone pair electrons from the enol reform the carbonyl C=O bond and promoted the elimination of water as a leaving group.
5) Deprotonation
Deprotonation by water in the final step create the neutral enone product and regenerates the acid catalyst.
Aldol Condensation
Whether an aldol reaction or an aldol condensation product is formed during a reaction largely depends on the reaction conditions. Typically, a reaction with a base at room temperature provides the aldol reaction product. However, if the reaction mixture is heated the aldol product is quickly converted into the aldol condensation product. If the condensation product is desired the aldol intermediate is usually not isolated.
Examples
Aldol Reaction
Aldol Condensation
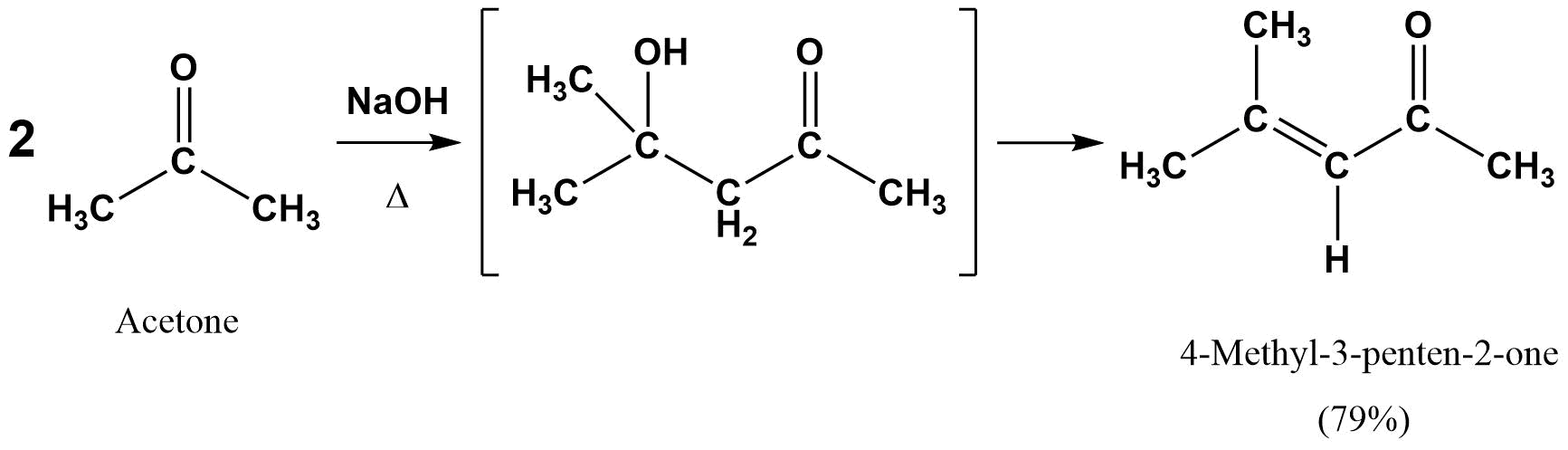
Draw the product of an aldol condensation with the following molecule:
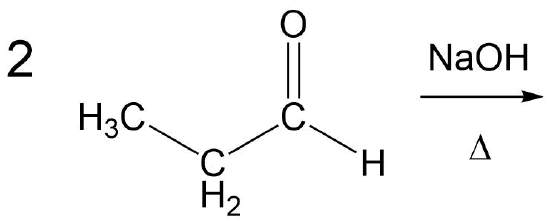
- Answer
-
The overall reaction is a combination of two major steps, an aldol reaction followed by a dehydration to form the enone. In this situation it is best to consider the aldol product first (as discussed in Section 23.3, then convert it to the enone. Note! The double bond always forms in conjugation with the carbonyl.

