3.1: Preparing Ethers
- Page ID
- 469368
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Ether Formation Though Dehydration
Acid-catalyzed dehydration of small 1º-alcohols constitutes a specialized industrial method of preparing symmetrical ethers. This reaction cannot be employed to prepare unsymmetrical ethers because a mixture of products is likely to be obtained. Also, 2o and 3o alcohols cannot be used for this reaction because they dehydrate to form alkenes by an E1 mechanism.
\[\ce{2 CH_3CH_2-OH + H_2SO_4 ->[130\;^oC] CH_3CH_2\bond{-}O\bond{-}CH_2CH_3 + H_2O} \tag{18.2.1} \]
Mechanism
In the first step of the reaction mechanism, one alcohol is protonated to become a good leaving group. In the second step, a second alcohol displaces water from the protonated alcohol during an SN2 reaction yielding a protonated ether. In the final step, this intermediate is deprotonated to yield the symmetrical ether.
Williamson Ether Synthesis
One important procedure, known as the Williamson Ether Synthesis, proceeds by an SN2 reaction of an alkoxide nucleophile with a primary alkyl halide or tosylate. As previously discussed in Section 17-2, alkoxides are commonly created by deprotonating an alcohol with a strong base, such as sodium hydride (NaH). Simple alcohols can be used a solvent during a Williamson ether synthesis and with their alkoxide created through the addition of sodium metal (Na(s)).
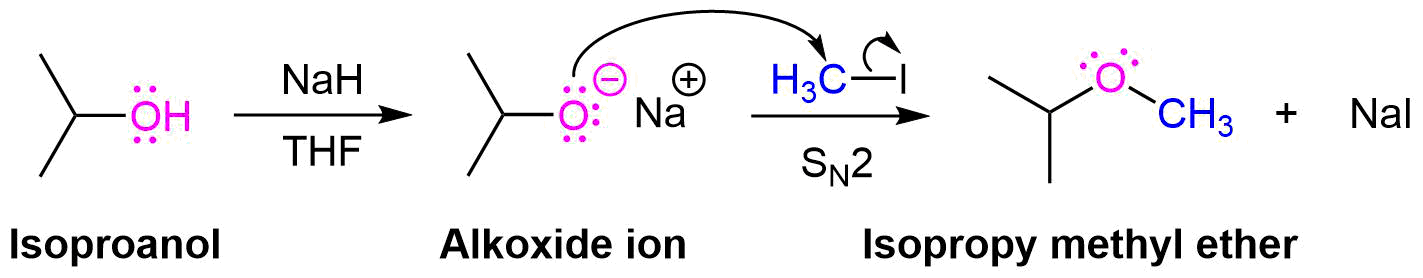
Planning a Williamson Ether Synthesis
The Williamson ether synthesis has the same limitations as other SN2 reactions, as discussed in Section 11-3. Since alkoxide anions are strong bases, utilizing 2o or 3o halogen leaving groups could possibly produce an E2 elimination product. When considering the synthesis of an unsymmetrical ether, there are two different combinations of reactants possible and each should be carefully considered. In general, the pathway which utilizes the least sterically hindered halogen will be preferred.
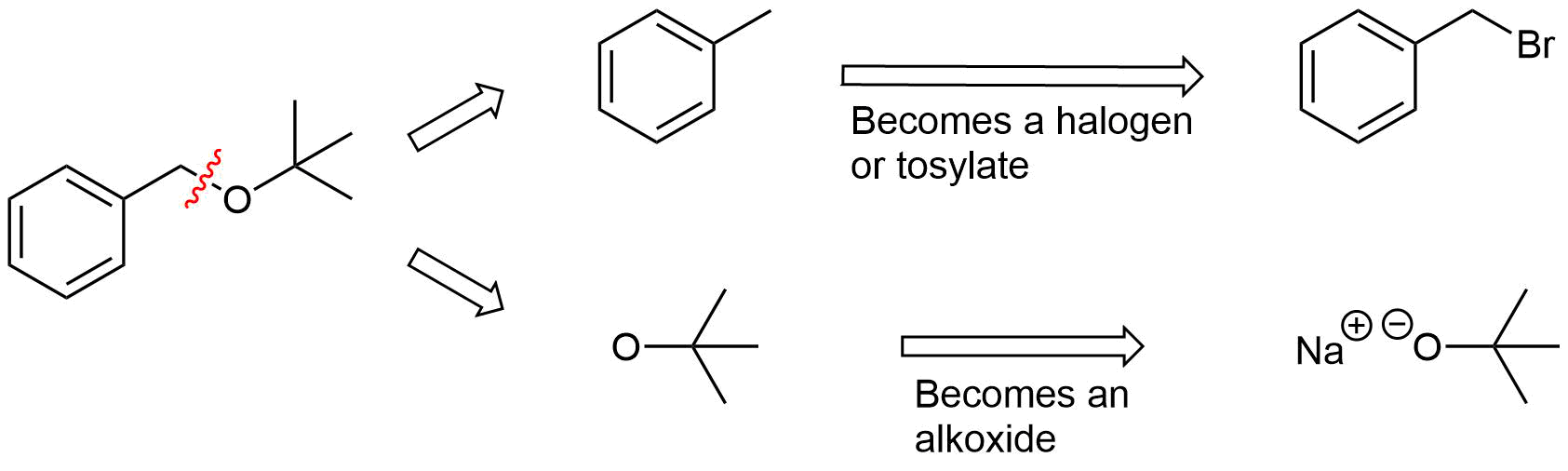
The key bond cleavage in the target molecule involves a C-O bond. Because unsymmetrical ethers have two unique C-O bonds, each can be broken to provide a unique set of reactants. After cleavage, the fragment with the oxygen will become an alkoxide. The other fragment will become a halogen or tosylate.
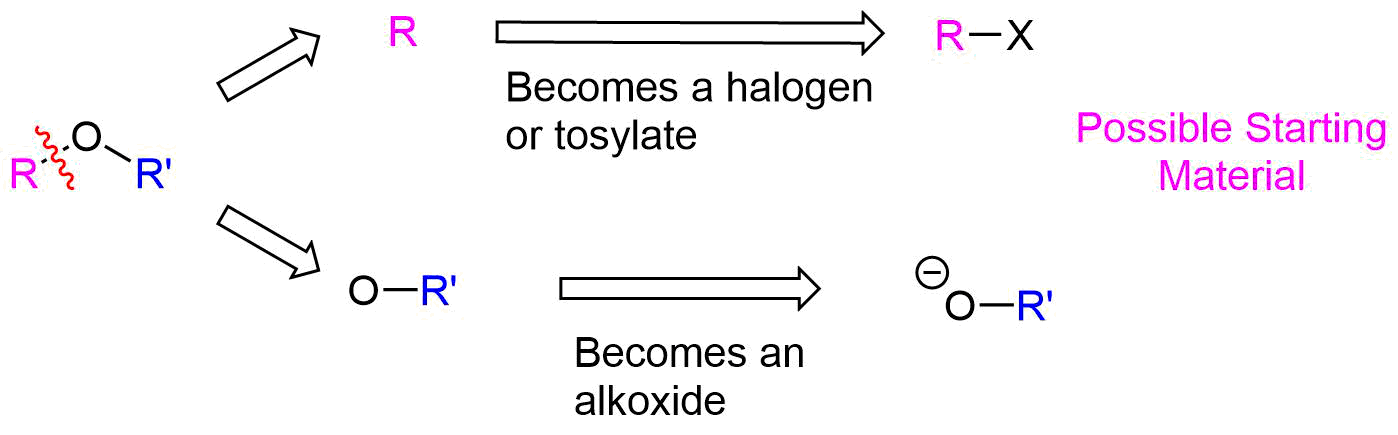
How would you prepare the following molecule using a Williamson Ether Synthesis?
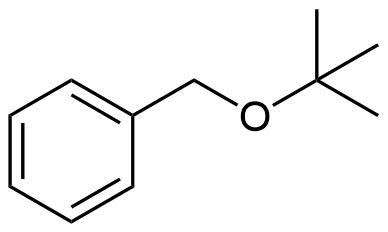
- Answer
-
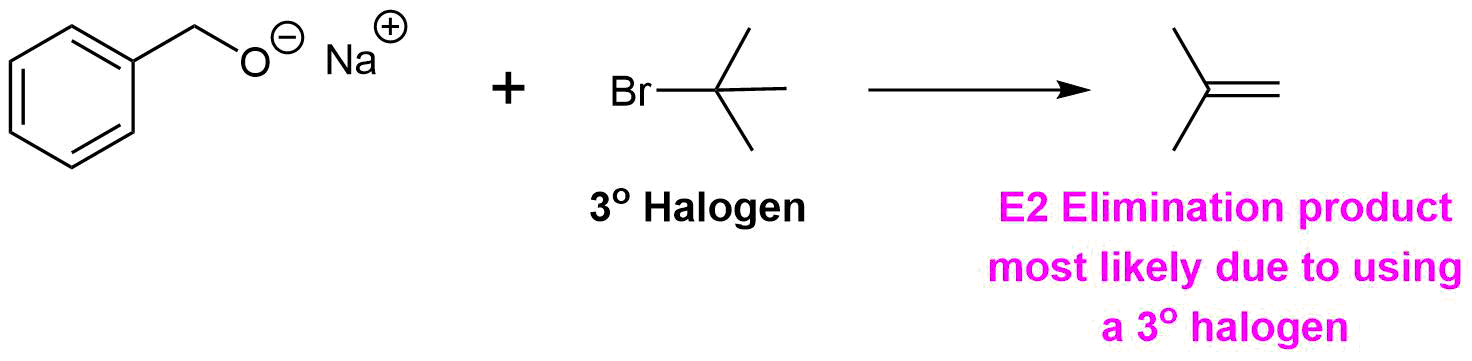
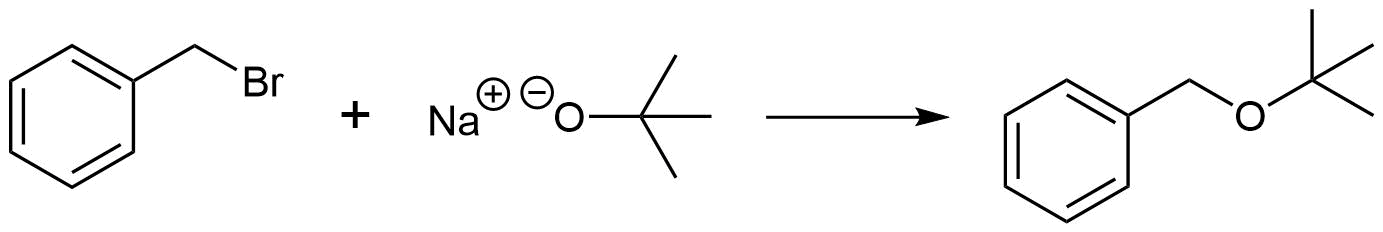
Analysis: The ether is asymmetrical so each of the C-O bonds can be broken to create a different set of possible reactants. After cleavage of the C-O bond, pathway 1 shows a 3o halogen as the starting material. This reaction will most likely not be effective due to alkoxides reacting with 3o halogens to preferable form an alkenes by E2 elimination. Pathway 2 shows a 1o halogen as a starting material which is favorable for SN2 reactions.
Pathway 1
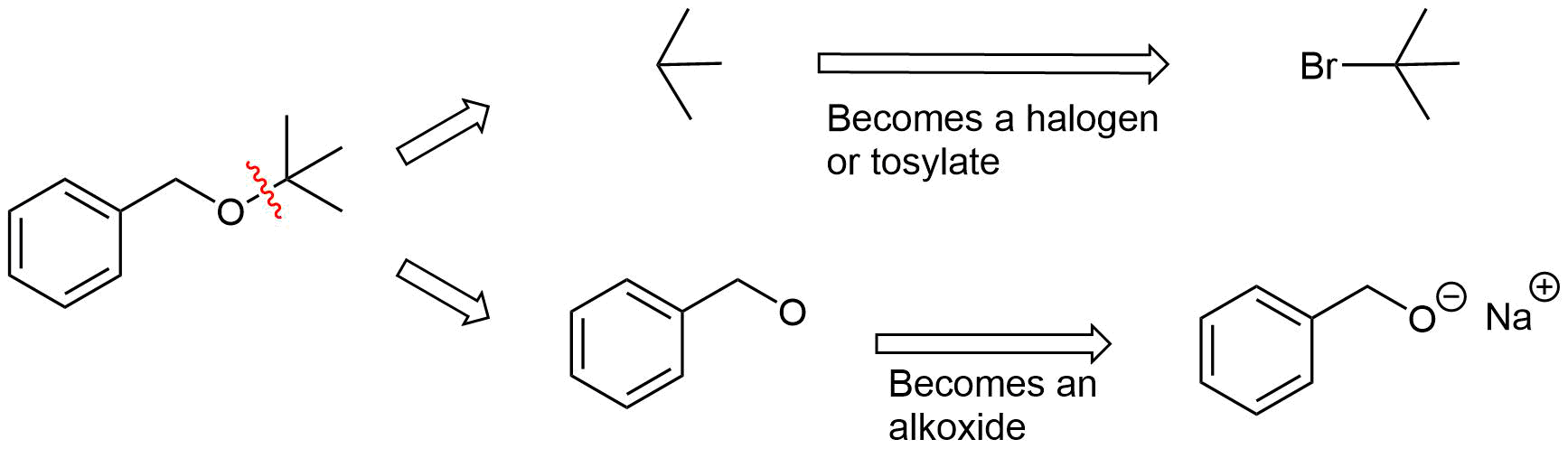
Solution 1
Pathway 2
Solution 2
Contributors and Attributions
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Layne Morsch (University of Illinois Springfield)

