7.3: Question 7.4.6 PASS - identifying constitutional isomers
- Page ID
- 452283
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Indicate whether each of the following sets are constitutional isomers, the same compound, or different compounds.
a) 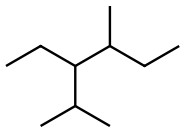 &
& 
b) 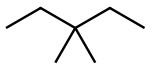 &
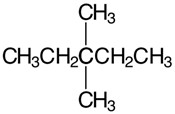
& 
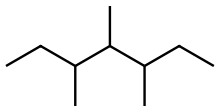
c)  &
& 
d)  &
& 
- Answers
-
a) Constitutional isomers
b) Same compound
c) Constitutional isomers
d) Different compounds
See LibreText 7.4 Alkanes and Alkane Isomers
- Strategy Map
-
Step Hint 1. Identify the atoms that make up each compound (their chemical formula). 2. Identify the connectivity of each compound. Recall the different ways to draw chemical structures and what line drawings of organic compounds represent (see LibreText section 7.2):
3. Compare the two compounds and identify what is the same/different between the two.
- Solution
-
a) Both structures have the same formula but have different connectivity which makes them constitutional isomers.
b) Both structures have the same formula and the same connectivity which makes them the same compound.
c) Both structures have the same formula but have different connectivity which makes them constitutional isomers.
d) The structures have different formulas which makes them different compounds.
- Guided Solution
-
Download Guided Solution as a pdf
Guided Solution Hint This is a theory type problem where you must identify structural isomers based on their chemical formula and connectivity. See LibreText 7.4 Alkanes and Alkane Isomers Question: Indicate whether each of the following sets are constitutional isomers, the same compound, or different compounds.
Recall the different ways we can draw chemical structures. See LibreText 7.2 Drawing Chemical Structures Recall what a constitutional isomer is. Constitutional isomers are two or more molecules that have the same chemical formula (are composed of the same atoms), but have different connectivity.
For example, the methane group is attached to carbon four on one compound and carbon six on another, but both have the same chemical compounds.
What will make the two compounds different compounds? The two compounds are considered different compounds if they have a different arrangement or connectivity of atoms. Complete Solution:
a) Count the C and H atoms. We count Count the C and H atoms. We count seven C and sixteen H for each structure. Both structures therefore have the molecular formula. Both structures therefore have the molecular formula C10H22. Looking closely you can see the atoms are connected differently, which makes them constitutional isomers.
Answer constitutional isomers
b) Count the C and H atoms. We count seven C and sixteen H for each structure. Both structures therefore have the molecular formula C7H16 and they have the same connectivity which makes them the same compound.
Answer same compound
c) Count the C and H atoms. We count seven C and sixteen H for each structure. Both structures therefore have the molecular formula of C7H16 but looking closely we can see they have different connectivity which makes them constitutional isomers.
Answer constitutional isomers
d) Count the C and H atoms. We count nine C and twenty H for the left structure and count ten C and twenty-two H for the right structure. The structure on the left has a molecular formula of C9H20 and the structure on the right has a formula of C10H22 which makes them different compounds.
Answer different compounds
 &
& 
Notice that the C chains in the two structures are different.
 &
& 
These are the same just different ways to represent the structure.
 &
& 
Notice the different position of one of the C branches making the connectivity different even though the atoms are the same.
 &
& 
C9H20 C10H22
Check your work!
Reviewing your work above checking the connectivity and number of atoms will confirm your answers.
Why do these answers make chemical sense?
The formula of compounds is determined by the atoms that compose them. Multiple compounds can have the same chemical makeup and formula, but their connectivity may be different. If this is the case, they will be constitutional isomers as in (a) and (c) above. This differentiation is made because they will often have different chemical properties. If the number of atoms and connectivity is the same then they will be the same compound as in (b) where you are comparing a line structure drawing to a condensed structural formula. If the number of atoms are different as in (d) the molecular formula is different and they are different compounds.
(question source from page titled 3.2: Alkanes and Alkane Isomers https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/03%3A_Organic_Compounds-_Alkanes_and_Their_Stereochemistry/3.02%3A_Alkanes_and_Alkane_Isomers shared under a CC BY 4.0 and was authored, remixed, and/or curated by Steven Farmer, Dietmar Kennepohl, Zachary Sharrett, Layne Morsch, Krista Cunningham, & Tim Soderberg.)


