4.2: Question 4.E.60 PASS - draw Lewis Structure and calculate formal charge
- Page ID
- 452249
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Iodine forms a series of fluorides (listed here). Draw Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule:
a) IF
b) IF3
c) IF5
- Answer
-
Lewis Structures:
a)
 b)
b) 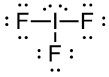
c)
 d)
d) 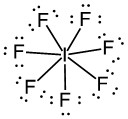
Formal charges on iodine:
a) IF, formal charge on I = 0
b) IF3, formal charge on I = 0
c) IF5, formal charge on I = 0
d) IF7, formal charge on I = 0
See LibreText 4.4 Lewis Symbols and Structures and LibreText 4.5 Formal Charges and Resonance
- Strategy Map
-
Step Hint 1. Count valence electrons in the molecule. Valence electrons are the outer shell electrons. 2. Draw the Lewis Structure:
- Pick your center atom (if applicable) by selecting the atom that has the lowest electronegativity.
- Arrange and connect your atoms. Start with a single bond represented by a single line between connected atoms.
- Add lone pairs on terminal atoms until you have used them all.
- Check that you followed the octet rule and that the total number of electrons you used (either as a dot/lone pair or in a line) add up to the total amount you counted in the beginning.
Recall your steps for drawing Lewis Structures (see LibreText section 4.4)
If you have left over electrons and your center atom's octet is full, check and see if that center atom can have an expanded octet.
3. Calculate formal charge:
- Identify the number of valence electrons the atom(s) of interest have.
- Using your drawn Lewis Structure identify the number of valence electrons assigned to the atom(s) of interest.
- Subtract your assigned electrons from the original valence electrons for each atom of interest. Charges can be positive or negative, zero means there is no formal charge for that atom.
Recall your steps to determine formal charge (see LibreText section 4.5)
In a Lewis Structure, the assigned electrons are its surrounding lone pairs (one electron per dot, two electrons per pair) as well as half of the electrons from each line (this is because lines represent shared electrons, so each atom is assigned one from that pair).
- Solution (a) IF
-
Count valence electrons:
(1) Iodine + (1) Fluorine
7ve- + 7ve- = 14 valence electrons
Draw Lewis Structure:

Formal Charge on I:
Valence electrons
7
Assigned electrons
7
Formal Charge
0
Answer: The formal charge on I is 0.
- Solution (b) IF3
-
Count valence electrons:
(1) Iodine + (3) Fluorine
7ve- + 3(7ve-) = 7 + 21 = 28 valence electrons
Draw Lewis Structure:
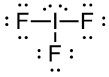
Formal Charge on I:
Valence electrons
7
Assigned electrons
7
Formal Charge
0
Answer: The formal charge on I is 0.
- Guided Solution (b) IF3
-
Download Guided Solution as a pdf
Guided Solution Hint This is a Lewis Structure application question. In this problem, you must follow the steps to create four different Lewis Structures and find their corresponding formal charges. See LibreText 4.4 Lewis Symbols and Structures and LibreText 4.5 Formal Charges and Resonance Iodine forms a series of fluorides (listed here). Draw Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule:
b) IF3
Count valence electrons in the molecule by identifying what atoms there are, counting the valence electrons each has and adding them. To find how many valence electrons an atom has, count across the periodic table (skipping the D block elements). You can use the Group number or electron configuration to find the number of valence electrons in each type of atom.
Group 1=1 Group 2=2 Group 13=3 Group 14=4 Group 15=5 Group 16=6 Group 17=7 Group 18=8 (full outer shell) You cannot have more electrons in a molecule than the amount each of the atoms had combined. For #1 Iodine has 7 valence electrons and Fluorine has 7 valence electrons, so the total number of electrons in the structure of IF will be 14.Draw the Lewis Structure:
- Pick your center atom (if applicable) by selecting the atom that has the lowest electronegativity.
- Arrange and connect your atoms. Start by drawing all your atoms and connecting them with one line, this line represents a single bond and is your “bare-bones step” showing how the atoms are arranged. Each line represents two shared electrons.
- Add lone pairs on terminal atoms until you have used them all, or until each atom has a full octet.
- In the case that you run out of electrons before all octets are full, make double or triple bonds between sharing atoms.
- In the case that you have remaining electrons, add them to the center atom until its octet is full.
- Check that you followed the octet rule and that the total number of electrons you used (either as a dot/lone pair or in a line) add up to the total amount you counted in the beginning.
In our case I has a lower electronegativity, and F can only form one bond, so I will be the central atom and will be bonded to each F.
Sometimes it can be confusing where to add a double bond. If you run out of available electrons, and some of your atoms still remain without a full octet, you can add a double bond between two or more atoms as long as it still abides by the octet rule. If you have left over electrons and your center atom's octet is full, check and see if that center atom can have an expanded octet.
Calculate formal charge:
- Identify the number of valence electrons the atom(s) of interest have and add them up for each atom.
- Using your drawn Lewis Structure identify the number of valence electrons assigned to the atom(s) of interest.
- Subtract your assigned electrons from the original valence electrons for each atom of interest. Charges can be positive or negative, zero means there is no formal charge for that atom.
You counted these up to be able to draw your Lewis Strucrure.
In a Lewis Structure, the assigned electrons are its surrounding lone pairs (one electron per dot, two electrons per pair) as well as half of the electrons from each line (this is because lines represent shared electrons, so each atom is assigned one from that pair).
It can be useful to track valence electrons and calculate formal charges using a table as shown below, especially if you are working with many atoms:
Element
(Element name)
(Element name)
Valence electrons (ve-)
#
#
Assigned electrons (ae-)
#
#
Formal Charge
(ve- - ae-)
#
#
Complete Solution b) IF3
Count valence electrons:
(1) Iodine + (3) Fluorine
Iodine has 7 valence e-
Each Fluorine has 7 valence e- and we have three F
7ve- + 3x(7ve- )= 28 total valence electrons
Draw Lewis Structure:
Connect I and each F by a line representing a single bond.
This has used 6 e-, and we have 22 left.
Add lone pairs to each F atom. This adds 3 lone pairs to each F to complete their octet, for a total of 18 more electrons used. We have 4 electrons left. Add them as two lone pairs to the central I. This has used the remaining electrons.
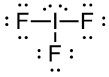
Determine Formal Charge:
Element
Iodine
Valence electrons
7
Assigned electrons
7
Formal Charge
(7 - 7)
0
Answer: The formal charge on I in IF3 is 0.
(Amount of Iodine atoms)x7Ve- + (amount of fluorine atoms)x7ve-
= total valence electrons
Each F atom has a complete octet so the octet rule is satisfied. I can have an expanded octet since it is in period 5.
Looking back at the Lewis Structure assign one electron to I from the single bond, and count up all the electrons in the lone pairs on I.
Check your work!
The formal charge on a neutral molecule should always be zero. IF3 is neutral so the zero formal charge is correct.
Why does this answer make chemical sense?
Lewis Structures are a visual representation of the electrons from an atom's outermost electron shell, to illustrate when they are shared in covalent bonds or present as lone pairs.
Formal charges occur when the number of electrons an atom has is different from the amount it originated with prior to the reaction. A formal charge can be either positive or negative. Positive if there are less assigned than previously had, negative if more are assigned than previously had. This makes sense as electrons carry a negative charge.
Formal charges can also be used to help decide which of multiple structures is preferred. See LibreText 4.5 (section 4.5.2 Using Formal Charge to Predict Molecular Structure)
(question source from page titled 7.E:Chemical Bonding and Molecular Geometry (Exercises): https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_1e_(OpenSTAX)/07%3A_Chemical_Bonding_and_Molecular_Geometry/7.E%3A_Chemical_Bonding_and_Molecular_Geometry_(Exercises), shared under a CC BY 4.0 license, authored, remixed, and/or curated by OpenStax, original source https://openstax.org/books/chemistry-2e/pages/7-exercises, Access for free at https://openstax.org/books/chemistry/pages/1-introduction)
- link to PASS Lewis Structure H5P Activity


