1.1: What is chemistry
- Page ID
- 367404
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Outline the historical development of chemistry
- Provide examples of the importance of chemistry in everyday life
- Describe the scientific method and informs the scientific process
Throughout human history, people have tried to convert matter into more useful forms. Our Stone Age ancestors chipped pieces of flint into useful tools and carved wood into statues and toys. These endeavors involved changing the shape of a substance without changing the substance itself. But as our knowledge increased, humans began to change the composition of the substances as well—clay was converted into pottery, hides were cured to make garments, copper ores were transformed into copper tools and weapons, and grain was made into bread.
Humans began to practice chemistry when they learned to control fire and use it to cook, make pottery, and smelt metals. Subsequently, they began to separate and use specific components of matter. A variety of drugs such as aloe, myrrh, and opium were isolated from plants. Dyes, such as indigo and Tyrian purple, were extracted from plant and animal matter. Metals were combined to form alloys—for example, copper and tin were mixed together to make bronze—and more elaborate smelting techniques produced iron. Alkalis were extracted from ashes, and soaps were prepared by combining these alkalis with fats. Alcohol was produced by fermentation and purified by distillation.
Attempts to understand the behavior of matter extend back for more than 2500 years. As early as the sixth century BC, Greek philosophers discussed a system in which water was the basis of all things. You may have heard of the Greek postulate that matter consists of four elements: earth, air, fire, and water. Subsequently, an amalgamation of chemical technologies and philosophical speculations were spread from Egypt, China, and the eastern Mediterranean by alchemists, who endeavored to transform “base metals” such as lead into “noble metals” like gold, and to create elixirs to cure disease and extend life (Figure 1).

From alchemy came the historical progressions that led to modern chemistry: the isolation of drugs from natural sources, metallurgy, and the dye industry. Today, chemistry continues to deepen our understanding and improve our ability to harness and control the behavior of matter. This effort has been so successful that many people do not realize either the central position of chemistry among the sciences or the importance and universality of chemistry in daily life.
Chemistry: The Central Science
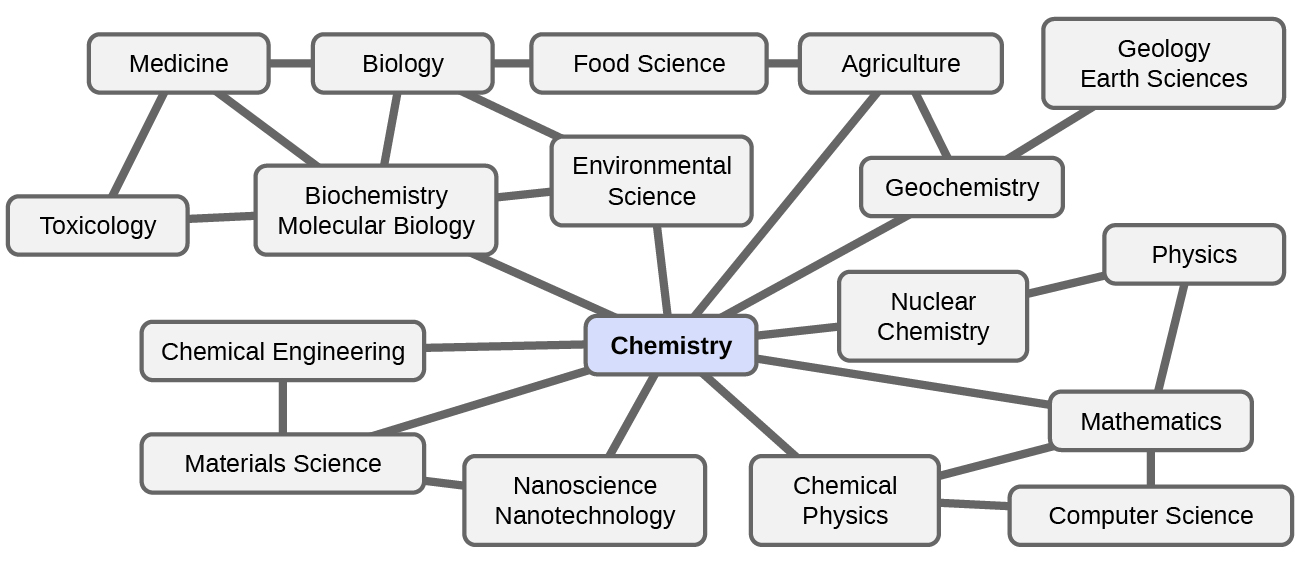
Chemistry is sometimes referred to as “the central science” due to its interconnectedness with a vast array of other STEM disciplines (STEM stands for areas of study in the science, technology, engineering, and math fields). Chemistry and the language of chemists play vital roles in biology, medicine, materials science, forensics, environmental science, and many other fields (Figure 2). The basic principles of physics are essential for understanding many aspects of chemistry, and there is extensive overlap between many sub disciplines within the two fields, such as chemical physics and nuclear chemistry. Mathematics, computer science, and information theory provide important tools that help us calculate, interpret, describe, and generally make sense of the chemical world. Biology and chemistry converge in biochemistry, which is crucial to understanding the many complex factors and processes that keep living organisms (such as us) alive. Chemical engineering, materials science, and nanotechnology combine chemical principles and empirical findings to produce useful substances, ranging from gasoline to fabrics to electronics. Agriculture, food science, veterinary science, and brewing and wine making help provide sustenance in the form of food and drink to the world’s population. Medicine, pharmacology, biotechnology, and botany identify and produce substances that help keep us healthy. Environmental science, geology, oceanography, and atmospheric science incorporate many chemical ideas to help us better understand and protect our physical world. Chemical ideas are used to help understand the universe in astronomy and cosmology.
What are some changes in matter that are essential to daily life? Digesting and assimilating food, synthesizing polymers that are used to make clothing, containers, cookware, and credit cards, and refining crude oil into gasoline and other products are just a few examples. As you proceed through this course, you will discover many different examples of changes in the composition and structure of matter, how to classify these changes and how they occurred, their causes, the changes in energy that accompany them, and the principles and laws involved. As you learn about these things, you will be learning chemistry, the study of the composition, properties, and interactions of matter. The practice of chemistry is not limited to chemistry books or laboratories: It happens whenever someone is involved in changes in matter or in conditions that may lead to such changes.
The Scientific Method
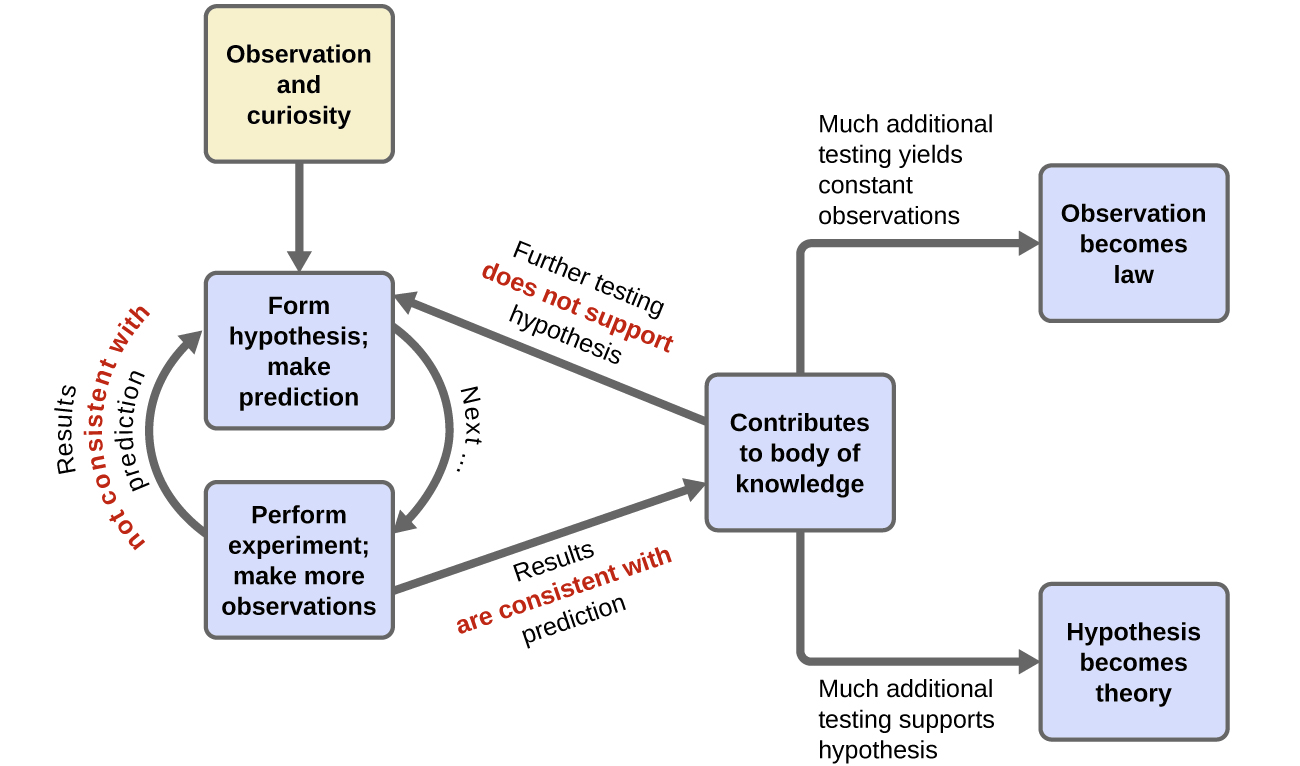
Chemistry is a science based on observation and experimentation. Doing chemistry involves attempting to answer questions and explain observations in terms of the laws and theories of chemistry, using procedures that are accepted by the scientific community. There is no single route to answering a question or explaining an observation, but there is an aspect common to every approach: Each uses knowledge based on experiments that can be reproduced to verify the results. Some routes involve a hypothesis, a tentative explanation of observations that acts as a guide for gathering and checking information. We test a hypothesis by experimentation, calculation, and/or comparison with the experiments of others and then refine it as needed.
Some hypotheses are attempts to explain the behavior that is summarized in laws. The laws of science summarize a vast number of experimental observations, and describe or predict some facet of the natural world. If such a hypothesis turns out to be capable of explaining a large body of experimental data, it can reach the status of a theory. Scientific theories are well-substantiated, comprehensive, testable explanations of particular aspects of nature. Theories are accepted because they provide satisfactory explanations, but they can be modified if new data become available. The path of discovery that leads from question and observation to law or hypothesis to theory, combined with experimental verification of the hypothesis and any necessary modification of the theory, is called the scientific method (Figure 3).
Key Takeaway
- Chemistry deals with the composition, structure, and properties of matter, and the ways by which various forms of matter may be inter converted.
- Chemists use the scientific method to perform experiments, pose hypotheses, and formulate laws and develop theories, so that they can better understand the behavior of the natural world.
- Chemists measure, analyze, purify, and synthesize a wide variety of substances that are important to our lives.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).

