11.2: Acid Strength
- Page ID
- 33982
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)All acids and bases do not ionize or dissociate to the same extent. This leads to the statement that acids and bases are not all of equal strength in producing H+ and OH- ions in solution. The terms "strong" and "weak" give an indication of the strength of an acid or base. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. If the acid or base conducts electricity strongly, it is a strong acid or base. If the acid or base conducts electricity weakly, it is a weak acid or base.
Demonstration of Acid and Base Conductivity
The instructor will test the conductivity of various solutions with a light bulb apparatus. The light bulb circuit is incomplete. If the circuit is completed by a solution containing a large number of ions, the light bulb will glow brightly indicating a strong ability to conduct electricity as shown for HCl. If the circuit is completed by a solution containing large numbers of molecules and either no ions or few ions, the solution does not conduct or conducts very weakly as shown for acetic acid.
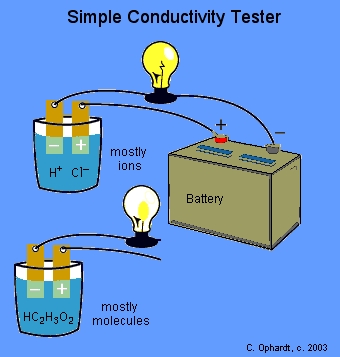
An acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acid or base which conducts electricity only weakly contains only a few ions and is called a weak acid or base.
| Compounds | Appearance of light bulb | Classification Weak or Strong |
Inference of Ions or Molecules |
|---|---|---|---|
| H2O | no light | weak | molecules |
| HCl | bright | strong | ions |
| HC2H3O2 | dim | weak | molecules |
| H2SO4 | bright | ||
| H2CO3 | dim | ||
| NaOH | bright | ||
| KOH | bright | ||
| NH4OH | dim |
Bond Strength
The bond strengths of acids and bases are implied by the relative amounts of molecules and ions present in solution. The bonds are represented as:
| acid | base |
| H-A | M-OH |
where A is a negative ion, and M is a positive ion
- Strong acids have mostly ions in solution, therefore the bonds holding H and A together must be weak. Strong acids easily break apart into ions.
- Weak acids exist mostly as molecules with only a few ions in solution, therefore the bonds holding H and A together must be strong. Weak acids do not readily break apart as ions but remain bonded together as molecules.
Bond Strength Principle
Acids or bases with strong bonds exist predominately as molecules in solutions and are called "weak" acids or bases. Acids or bases with weak bonds easily dissociate into ions and are called "strong" acids or bases.
| Characteristic | Strong Acid or Base | Weak Acid or Base |
|---|---|---|
| Molecules | few | large number |
| Ions | large number | small number |
| Conductivity | strong | weak |
| Bond Strength | weak | strong |
Acids and bases behave differently in solution based on their strength. Acid or base "strength" is a measure of how readily the molecule ionizes in water.
Introduction Again
Some acids and bases ionize rapidly and almost completely in solution; these are called strong acids and strong bases. For example, hydrochloric acid (HCl) is a strong acid. When placed in water, virtually every HCl molecule splits into a H+ ion and a Cl- ion in the reaction.1
\[\ce{HCl(aq) + H2O(l) <=> H3O^{+}(aq) + Cl^{-}(aq)} \nonumber\]
For a strong acid like HCl, if you place 1 mole of HCl in a liter of water, you will get roughly 1 mole of H30+ ions and 1 mole of Cl- ions. In a weak acid like hydrofluoric acid (HF), not all of the HF molecules split up, and although there will be some H+ and F- ions released, there will still be HF molecules in solution1. A similar concept applies to bases, except the reaction is different. A strong base like sodium hydroxide (NaOH) will also dissociate completely into water; if you put in 1 mole of NaOH into water, you will get 1 mole of hydroxide ions.1
\[\ce{NaOH(aq) + H2O(l) <=> Na^{+}(aq) + OH^{-}(aq) + H2O(l)} \nonumber\]
The terms "strong" and "weak" in this context do not relate to how corrosive or caustic the substance is, but only its capability to ionize in water. The ability of a substance to eat through other materials or damage skin is more of a function of the properties of that acid, as well as its concentration. Although, strong acids are more directly dangerous at lower concentrations a strong acid is not necessarily more dangerous than a weak one. For example, hydrofluoric acid is a weak acid1, but it is extremely dangerous and should be handled with great care. Hydrofluoric acid is particularly dangerous because it is capable of eating through glass, as seen in the video in the links sectionV1. The percent dissociation of an acid or base is mathematically indicated by the acid ionization constant (Ka) or the base ionization constant (Kb)1. These terms refer to the ratio of reactants to products in equilibrium when the acid or base reacts with water. For acids the expression will be
Ka = [H3O+][A-]/[HA]
where HA is the concentration of the acid at equilibrium, and A- is the concentration of its conjugate base at equilibrium and for bases the expression will be
\[K_b = \dfrac{[\ce{OH^{-}}][\ce{HB^{+}}]}{\ce{B}}\]
where B is the concentration of the base at equilibrium and HB+ is the concentration of its conjugate acid at equilibrium
The stronger an acid is, the lower the pH it will produce in solution. pH is calculated by taking the negative logarithm of the concentration of hydronium ions. For strong acids, you can calculate the pH by simply taking the negative logarithm of its molarity as it completely dissociates into its conjugate base and hydronium. The same goes for strong bases, except the negative logarithm gives you the pOH as opposed to the pH. For weak acids and bases, the higher the Ka or Kb, the more acidic or basic the solution. To find the pH for a weak acid or base, you must use the K equation and a RICE table to determine the pH.
All acids have a conjugate base that forms when they react with water, and similarly, all bases have a conjugate acid that reacts when they form with water.1 You can judge the relative strength of a conjugate by the \(K_a\) or \(K_b\) value of the substance because \(K_a \times K_b\) is equal to the ionization constant of water, Kw which is equal to \(1 \times 10^{-14}\) at room temperature. The higher the Ka, the stronger the acid is, and the weaker its conjugate base is. Similarly, the higher the Kb, the stronger the substance is as a base, and the more weakly acidic its conjugate acid is.1
Calculation of Ka
For an acid that reacts with water in the reaction
\[HA_{(aq)} + H_2O_{(l)} \rightleftharpoons H_3O^+_{(aq)} + A^-_{(aq)}\]
\[K_a = \dfrac{[H_3O^+][A^-]}{[HA]}\]
where each bracketed term represents the concentration of that substance in solution.
Relation of Kw, Kb, Ka
\[K_w = K_a \times K_b \nonumber\]
Partial List of Strong Acids: Hydrochlroic acid (HCl), Nitric Acid (HNO3), Perchloric Acid (HClO4), Sulfuric Acid (H2SO4)
Partial List of Strong Bases: Sodium Hydroxide (NaOH), Barium Hydroxide (Ba(OH)2), Calcium Hydroxide (Ca(OH)2), Lithium Hydroxide (LiOH) (Hydroxides of Group I and II elements are generally strong bases)
Partial List of Weak Acids: Acetic Acid (CH3COOH), Carbonic Acid (H2CO3), Phosphoric Acid (H3PO4)
Partial List of Weak Bases: Ammonia (NH3), Calcium Carbonate (CaCO3), Sodium Acetate (NaCH3COO)
Find the pH of 0.5 grams of HCl disolved into 100 ml of water:
Solution
First find moles of acid:
grams / molar mass = moles
0.5 grams / (36.5 g/mole) = 0.014 moles HCl
Then find molarity:
moles / volume = molarity
0.014 moles / 0.100 L = 0.14 M
HCl is a strong acid and completely dissociates in water, therefore the pH will be equal to the negative logarithm of the concentration of HCl
pH = -log(H3O+)
pH = -log(0.14) = 0.85
The Ka value for acetic acid is 1.76*10-5, and the Ka value for benzoic acid is 6.46*10-5, if two solutions are made, one from each acid, with equal concentrations, which one will have the lower pH?
Solution
The Ka value is a measure of the ratio between reactants and products at equilibrium. For an acid, the reaction will be HA + H2O --> A- + H3O+ . PH is based on the concentration of the hydronium ion (H3O+) which is a product of the reaction of acid and water. A higher Ka value means a higher ratio of reactants to products, and so the acid with the higher Ka value will be producing more hydronium, and therefore have a lower pH. Therefore the solution of benzoic acid will have a lower pH.
The Ka value of ammonium (NH4+) is 5.6*10-10, the Kb value of ammonia (NH3) 1.8*10-5, is ammonium more strongly acidic than ammonia is basic?
Solution
The relative strength of an acid or base depends on how high its Ka or Kb value is, in this case, the Ka value is far lower than the Kb value so the ammonia is more strongly basic than ammonium is acidic.
References
- Oxtboy, Gillis, Campion, David W., H.P., Alan. "Acid-Base Equilibria." Principles of Modern Chemistry. Belmont: Thomson Higher Education, 2008.
Contributors and Attributions
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook,. Lloyd McCarthy (UCD)

