8.1: Chemical Bonds, Lewis Symbols, and the Octet Rule
- Page ID
- 34915
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- To use Lewis electron dot symbols to predict the number of bonds an element will form.
Bonding Overview
Why are some substances chemically bonded molecules and others are an association of ions? The answer to this question depends upon the electronic structures of the atoms and nature of the chemical forces within the compounds. Although there are no sharply defined boundaries, chemical bonds are typically classified into three main types: ionic bonds, covalent bonds, and metallic bonds. In this chapter, each type of bond wil be discussed and the general properties found in typical substances in which the bond type occurs
- Ionic bonds results from electrostatic forces that exist between ions of opposite charge. These bonds typically involves a metal with a nonmetal
- Covalent bonds result from the sharing of electrons between two atoms. The bonds typically involves one nonmetallic element with another
- Metallic bonds These bonds are found in solid metals (copper, iron, aluminum) with each metal bonded to several neighboring groups and bonding electrons free to move throughout the 3-dimensional structure.
Each bond classification is discussed in detail in subsequent sections of the chapter. Let's look at the preferred arrangements of electrons in atoms when they form chemical compounds.
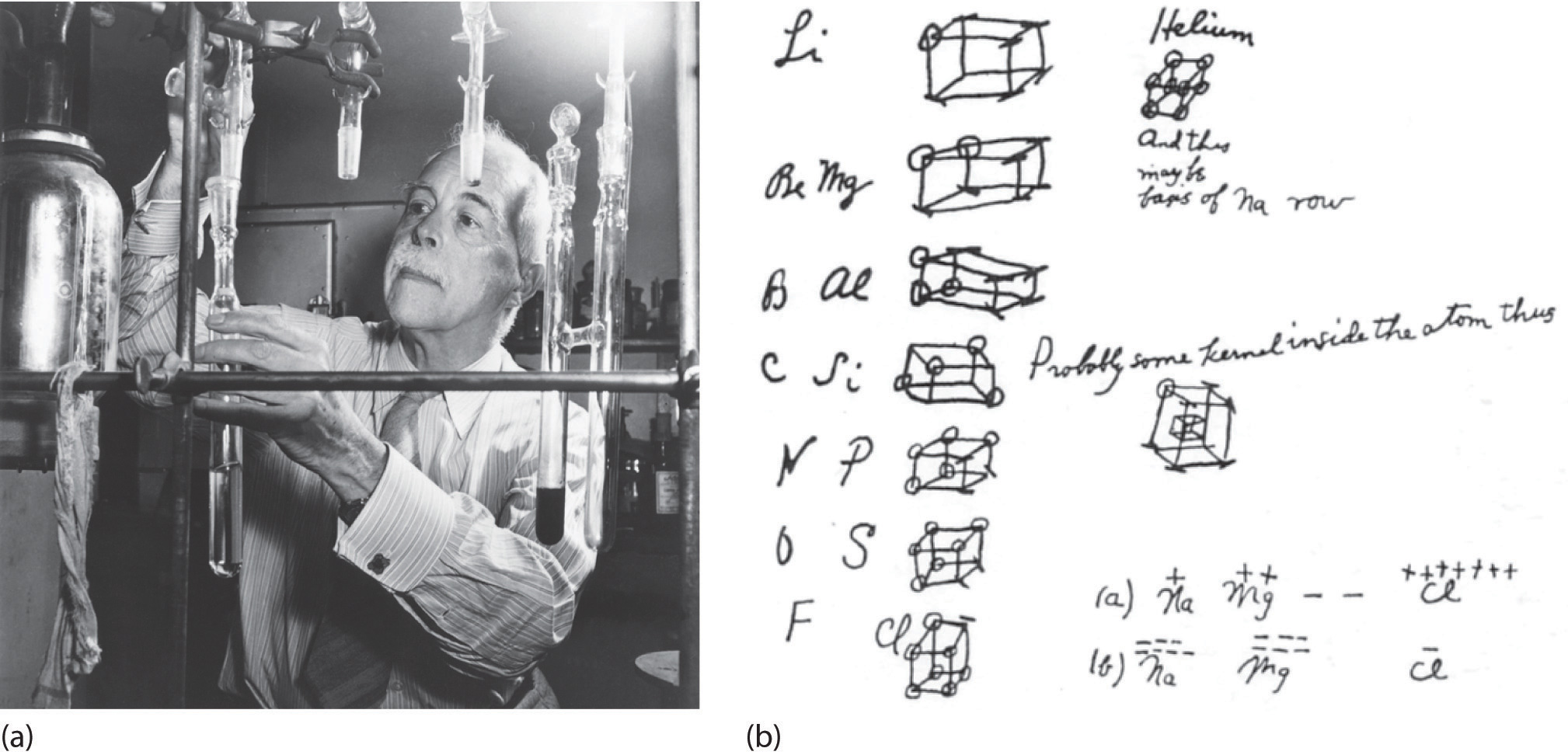
Figure 8.1.1 G. N. Lewis and the Octet Rule. (a) Lewis is working in the laboratory. (b) In Lewis’s original sketch for the octet rule, he initially placed the electrons at the corners of a cube rather than placing them as we do now.
Lewis Symbols
At the beginning of the 20th century, the American chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbols, often shortened to Lewis dot symbols—that can be used for predicting the number of bonds formed by most elements in their compounds. Each Lewis dot symbol consists of the chemical symbol for an element surrounded by dots that represent its valence electrons.
Note
Lewis Dot symbols:
- convenient representation of valence electrons
- allows you to keep track of valence electrons during bond formation
- consists of the chemical symbol for the element plus a dot for each valence electron
To write an element’s Lewis dot symbol, we place dots representing its valence electrons, one at a time, around the element’s chemical symbol. Up to four dots are placed above, below, to the left, and to the right of the symbol (in any order, as long as elements with four or fewer valence electrons have no more than one dot in each position). The next dots, for elements with more than four valence electrons, are again distributed one at a time, each paired with one of the first four. For example, the electron configuration for atomic sulfur is [Ne]3s23p4, thus there are six valence electrons. Its Lewis symbol would therefore be:
Fluorine, for example, with the electron configuration [He]2s22p5, has seven valence electrons, so its Lewis dot symbol is constructed as follows:
![]()
The number of dots in the Lewis dot symbol is the same as the number of valence electrons, which is the same as the last digit of the element’s group number in the periodic table. Lewis dot symbols for the elements in period 2 are given in Figure 8.1.2.
Lewis used the unpaired dots to predict the number of bonds that an element will form in a compound. Consider the symbol for nitrogen in Figure 8.1.2. The Lewis dot symbol explains why nitrogen, with three unpaired valence electrons, tends to form compounds in which it shares the unpaired electrons to form three bonds. Boron, which also has three unpaired valence electrons in its Lewis dot symbol, also tends to form compounds with three bonds, whereas carbon, with four unpaired valence electrons in its Lewis dot symbol, tends to share all of its unpaired valence electrons by forming compounds in which it has four bonds.
Figure 8.1.2: Lewis Dot Symbols for the Elements in Period 2
The Octet Rule
Lewis’s major contribution to bonding theory was to recognize that atoms tend to lose, gain, or share electrons to reach a total of eight valence electrons, called an octet. This so-called octet rule explains the stoichiometry of most compounds in the s and p blocks of the periodic table. We now know from quantum mechanics that the number eight corresponds to one ns and three np valence orbitals, which together can accommodate a total of eight electrons. Remarkably, though, Lewis’s insight was made nearly a decade before Rutherford proposed the nuclear model of the atom. An exception to the octet rule is helium, whose 1s2 electron configuration gives it a full n = 1 shell, and hydrogen, which tends to gain or share its one electron to achieve the electron configuration of helium.
Lewis dot symbols can also be used to represent the ions in ionic compounds. The reaction of cesium with fluorine, for example, to produce the ionic compound CsF can be written as follows:

No dots are shown on Cs+ in the product because cesium has lost its single valence electron to fluorine. The transfer of this electron produces the Cs+ ion, which has the valence electron configuration of Xe, and the F− ion, which has a total of eight valence electrons (an octet) and the Ne electron configuration. This description is consistent with the statement that among the main group elements, ions in simple binary ionic compounds generally have the electron configurations of the nearest noble gas. The charge of each ion is written in the product, and the anion and its electrons are enclosed in brackets. This notation emphasizes that the ions are associated electrostatically; no electrons are shared between the two elements.
Note
Atoms often gain, lose, or share electrons to achieve the same number of electrons as the noble gas closest to them in the periodic table.
As you might expect for such a qualitative approach to bonding, there are exceptions to the octet rule, which we describe elsewhere. These include molecules in which one or more atoms contain fewer or more than eight electrons.
Summary
- Lewis dot symbols can be used to predict the number of bonds formed by most elements in their compounds.
One convenient way to predict the number and basic arrangement of bonds in compounds is by using Lewis electron dot symbols, which consist of the chemical symbol for an element surrounded by dots that represent its valence electrons, grouped into pairs often placed above, below, and to the left and right of the symbol. The structures reflect the fact that the elements in period 2 and beyond tend to gain, lose, or share electrons to reach a total of eight valence electrons in their compounds, the so-called octet rule. Hydrogen, with only two valence electrons, does not obey the octet rule.

