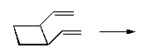A sigmatropic rearrangement which involves a [1,5] hydrogen shift, like the one shown below illustrates, a hydrogen atom (s orbital) moving across a 5 atom system containing two conjugated double bonds. Since there are three curved arrows in the mechanism, this is a 6 pi reaction. The rules for sigmatropic rearrangements discussed in the last section predict the hydrogen atom will undergo a suprafacial shift. The C-H bond breaking and the C-H bond forming, magenta dashed lines, are both on the bottom face of the molecule's pi system.
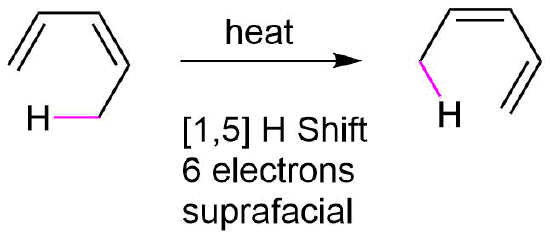
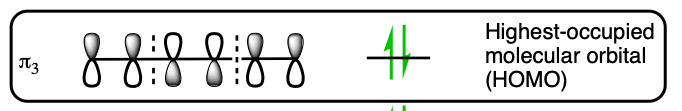
The suprafacial nature of this reaction comes from the orbital symmetry of the HOMO from a 6 pi electron molecular orbital. The orbital lobes on the terminal ends have the same sign on the same side.
During the reaction mechanism the hydrogen atom, an s orbital, is passes from one molecular to another of the same sign on the same side of the molecule or suprafacial.
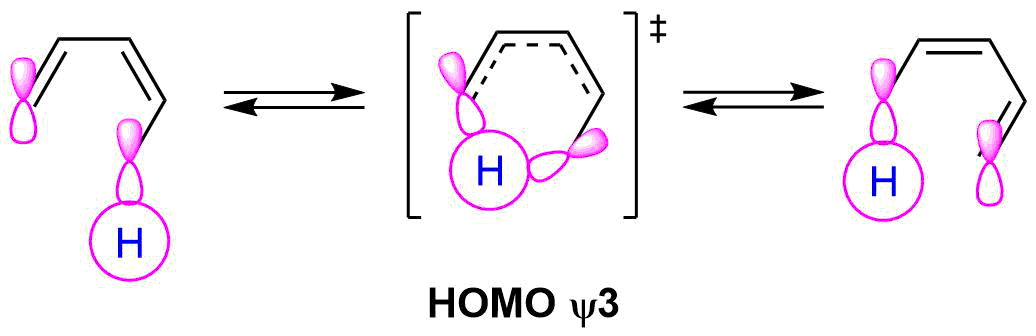
For example, 5-t-butyl-1,3-cyclopentadiene easily undergoes a [1,5] suprafacial shift of a hydrogen atom under thermal conditions to yield 1-t-butyl-1,3-cyclopentadiene as a rearranged product.

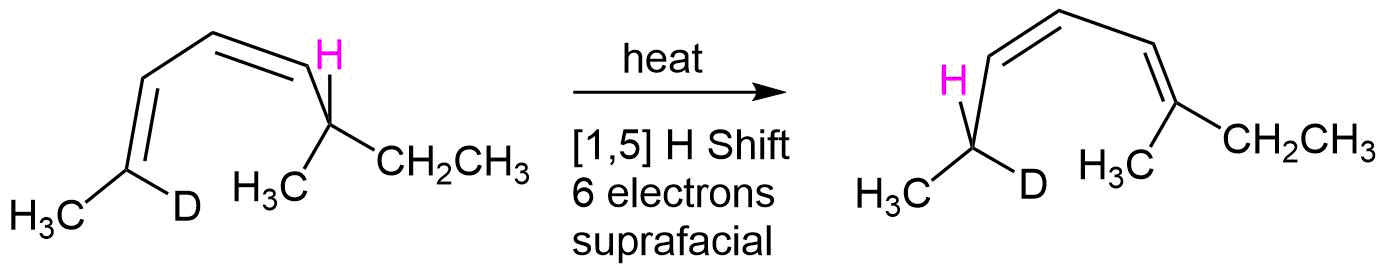
As another example, heating S-2-deuterio-6-methyl-(2E,4Z)-2,4-octadiene undergoes a thermal [1,5] suprafacial shift of a hydrogen to produce the product, R-2-deuterio-6-methyl-(2Z,4E)-3,5-octadiene with stereochemical control.
Claisen Rearrangement
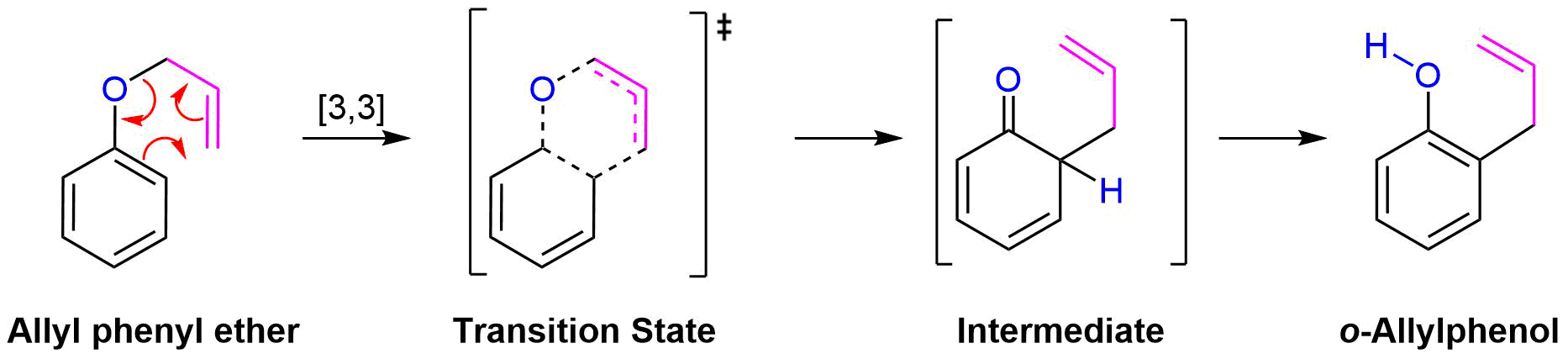
A very important example of a [3,3] sigmatropic reaction is the Claisen rearrangement of an allyl aryl ether or an allyl vinyl ether (Section 18-4). Heating an allyl aryl ether to 250 oC causes an sigmatropic rearrangement to produce an o-allylphenol. This rearrangement initially produces the non-aromatic 6-allyl-2,4-cyclohexadienone intermediate which quickly undergoes a proton shift to reform the aromatic ring in the o-allylphenol product. Claisen rearrangement occurs in a six-membered, cyclic transition state involving the movement of six bonding electron.
Allyl vinyl ethers can also undergo a Claisen rearrangement when heated to form gamma, delta -unsaturated ketones or aldehydes.
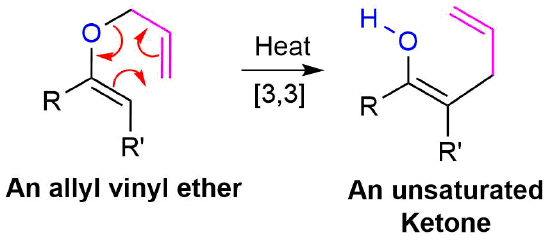
Cope Rearrangement
Another important example of a [3,3] sigmatropic reaction is the cope rearrangement. This reaction converts between isomers of a 1,5-hexadiene through a cyclic transition state.
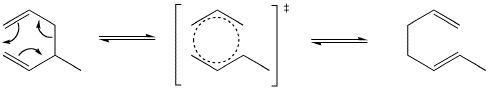
Both Cope and Claisen rearrangements involve the movement of six electrons which means they both react by suprafacial pathways under thermal conditions. These reactions can be considered to occur due to changes in overlap between orbitals around the cyclic transition state. Two orbitals which form the sigma bond being broken tilt away from each other while two orbitals that are pi bonding tilt toward each other to from a new sigma bond. After the change there are now two p orbitals parallel to each other on the left which then form new pi bonds.

Orbital rearrangement in a Cope rearrangement
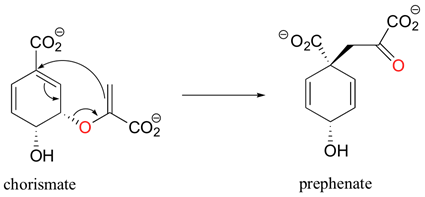
Sigmatropic rearrangements are rare in biological chemistry. One example is the chorismate mutase catalyzed Claisen rearrangement of chorismate (a allylic vinyl ether) to form prephenate. Prephenate is a precursor in the biosynthetic pathway of aromatic amino acids phenylalanine and tyrosine.
 .
.
Exercise \(\PageIndex{1}\)
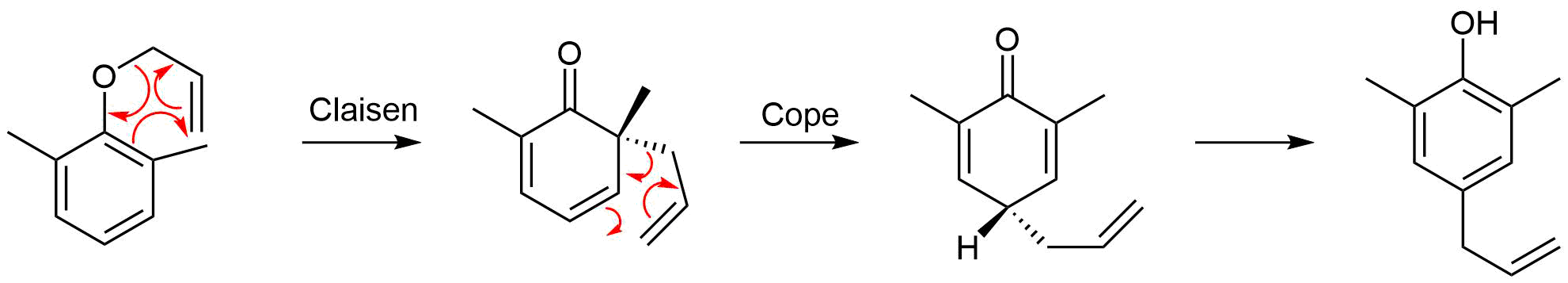
A Claisen rearrangement performed in the presence of ortho-substituents exclusively leads to para-substituted rearrangement products. This occurs via a Claisen rearrangement followed by a Cope rearrangement. Draw out the intermediates formed during this transformation and the mechanisms for both rearrangements.

- Answer
-
Exercise \(\PageIndex{2}\)
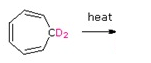
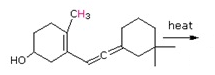
Draw the expected products of the following [1,5] suprafacial shifts.
a)
b)
- Answer
-
a)
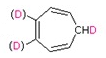
b)
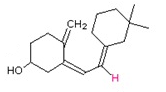
Exercise \(\PageIndex{3}\)
Draw the product of the following [3,3] suprafacial shifts.
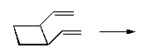
- Answer
-
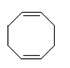


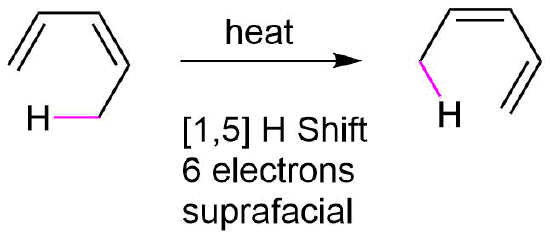

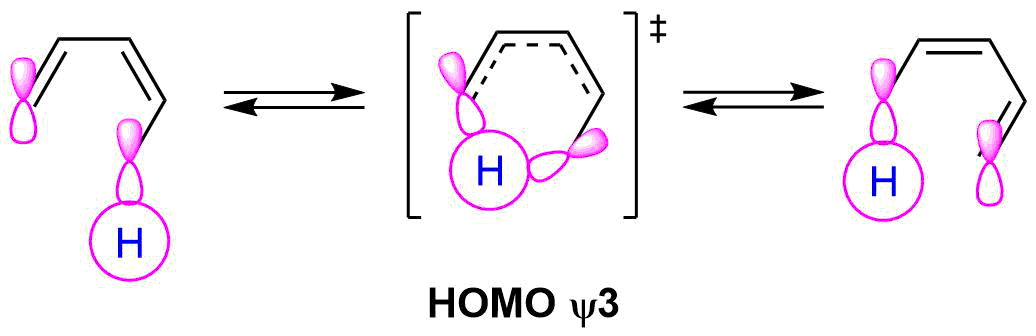



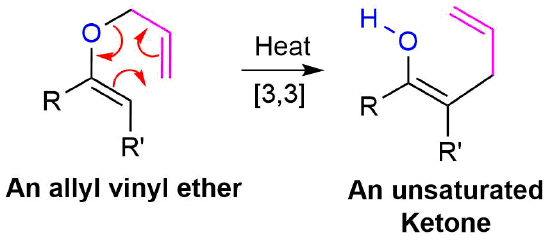


 .
.