12.2: Pd-Catalyzed Cross Coupling Reactions
- Page ID
- 447931
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to:
- Identify common Pd-catalyzed carbon-carbon bond forming reactions
- Draw and understand reaction mechanisms
- Use reactions in synthesis problems
Key Terms
Make certain that you can define, and use in context, the key terms below.
- Stille reaction
- Suzuki reaction
- Sonogashira reaction
- Heck reaction
Study Notes
Palladium catalyzed reactions are the most common transition metal catalyzed cross coupling reactions. Their importance was recognized with the 2010 Nobel Prize for Richard Heck, Ei-ichi Negishi, and Akira Suzuki. These reactions provide straightforward methods for the construction of Csp2-Csp2 and Csp2-Csp bonds. We will see many examples of these types of bond formations throughout this chapter. While learning this material, don't forget to contrast Pd catalyzed reactions with popular intro orgo carbon-carbon bond forming reactions like Grignard or organolithium reactions with carbonyls and epoxides. At least one component of these reactions was sp3 hybridized, so it was impossible to generate the types of bonds that are formed most commonly by Pd catalyzed reactions. Adding Pd catalyzed reactions to your organic chemistry toolbox will greatly expand the types of molecules that you are able to synthesize.
Content
This chapter will focus on learning and applying common Pd catalyzed reactions for the formation of Carbon-Carbon bonds: Stille, Suzuki, Sonogashira, and Heck. If you haven't already, please refer to the previous section for a discussion of transition metal structure and bonding along with common mechanistic steps in transition metal catalyzed reactions.
For all of the reactions in this chapter, we will depict the palladium catalyst as LnPd(0) to acknowledge that some number of ligands are bound to Pd but their actual number and structure are unimportant to the mechanism. Two things happen when a Pd complex dissolves in the reaction solvent. First, bonds to phosphine ligands break to establish an equilibrium with complexes having fewer ligands and open coordination sites for reactions to occur. Second, some reactions will begin with Pd(II) reagents. For example, most Sonogashira reactions are run with PdCl2(PPh3)2. These Pd reagents are reduced under the reaction conditions by several possible mechanisms that we won't concern ourselves with here. The net result is that Pd(0) is produced in the flask, and this complex can then catalyze the desired reactions. Even though we will generally ignore the phosphine ligands, the structure of these molecules is critically important to the success of Pd catalyzed reactions. Many of the recent advances in this field were made possible by the development of novel phosphine ligands.
Stille Reaction
The Stille reaction is the simplest Pd catalyzed cross coupling reaction where an organohalide combines with an organotin in the presence of a Pd(0) catalyst to form a new C-C bond. To avoid side reactions and production of mixtures, both components generally have Csp2 or Csp atoms bonded to the halogen and the tin. An example reaction and mechanism are shown below. Oxidative addition of bromobenzene with the Pd catalyst yields the first Pd(II) intermediate. Transmetallation transfers the alkene from Sn to Pd, generating the tributyltin bromide byproduct and the desired Pd(II) intermediate with two C-Pd bonds. Finally, reductive elimination generates the desired product and regenerates the Pd(0) catalyst.
Exercise \(\PageIndex{1}\)
Complete the following synthesis in 2 steps, one of which is a Stille reaction.
- Answer
-
Thinking retrosynthetically, we must be forming the single bond between the alkenes using a Stille reaction. That means we need to convert the ketone into a vinyl halide or halide equivalent in the first step. This is easily done by forming an enolate and adding triflic anhydride to make a vinyl triflate. As explained in the previous chapter, vinyl triflates participate readily in transition metal catalyzed reactions. So, our two step synthesis is 1) Deprotonation with LDA (to ensure we form the least substituted kinetic enolate) followed by addition of triflic anhydride to yield the vinyl triflate. 2) Stille reaction with vinyl tributyltin to generate the target.
Exercise \(\PageIndex{2}\)
Using retrosynthetic analysis, how would you make this target in one step using a Stille reaction?
- Answer
-
The key bond is the one between the alkene and the alkyne. This is the bond we will make in the forward direction using the Stille reaction.
Suzuki Reaction
The Suzuki reaction is similar to the Stille reaction but with boron used instead of tin for the transmetallation step. It also differs from the Stille reaction because Suzuki reactions require the presence of a base to activate the boron reagent prior to transmetallation. As shown below, the mechanistic steps are the same for these two popular transformations with the addition of the base promoted boron activation step that forms the reactive borate (negatively charged boron) compound. This borate reagent can be formed by a variety of oxygen bases in addition to carbonate like hydroxide and alkoxides (e.g., ethoxide, t-butoxide).
There are several advantages of the Suzuki reaction. First, we are already familiar with forming C-B bonds from intro orgo using a hydroboration reaction. Previously, we always followed this step with an oxidation reaction to form an alcohol. Now we can form the organoboron compound and use it in a Suzuki reaction. We can react alkenes or alkynes in hydroboration reactions to yield organoboron reagents for transmetallation. Several examples are shown below. Hydroboration of terminal alkynes is synthetically useful (internal alkynes yield product mixtures) when using hindered boranes like disiamylborane or catecholborane. Like all hydroborations, the reactions are syn additions with the larger boron adding to the less hindered side of the alkyne resulting in trans alkene products. To generate alkylboron reagents for use in Suzuki reactions, the most common reagent is 9-BBN (pictured below). A second advantage is the Suzuki reaction provides a way to use Pd catalysis to easily make Csp2-Csp3 or Csp-Csp3 bonds which can be challenging to make with other Pd catalyzed reactions. Hydroboration of an alkene provides the requisite B-Csp3 reagent (the 9-BBN derivative shown below) that participates in transmetallation then reductive elimination to yield the desired bond. We will see an example of this in one of the problems below.
Exercise \(\PageIndex{3}\)
What is the product of the follow reaction sequence?
- Answer
-
The first step is a hydroboration reaction with 9-BBN which is selective for the much less hindered terminal alkene. This sets up an example of a very synthetically useful intramolecular Suzuki reaction to form a 6-membered ring.
Exercise \(\PageIndex{4}\)
Propose a synthesis of the following target starting with compounds containing 6 carbons or fewer. One of your starting materials must be an alkyne.
- Answer
-
Thinking retrosynthetically, we can split the molecule between the two alkenes into two 6-carbon fragments that can be combined using a Suzuki reaction. The boron-containing component can then be simplified to the requisite alkyne starting material. In the forward direction, we start with 1-hexyne and hydroborate it with catecholborane (a 6-carbon reagent) to yield the vinylborane that participates in a Suzuki reaction with our 5-membered ring vinyl bromide to yield the target.
Sonogashira Reaction
The Sonogashira reaction enables the combination of an unsaturated carbon-containing halide or triflate with a terminal alkyne to yield a new Csp-Csp or Csp-Csp2 bond. One interesting aspect of this reaction is that it is catalyzed by a combination of Pd (to form the C-C bond) and Cu (to activate the terminal alkyne for transmetallation). Another key reagent is an amine base that promotes formation of the copper acetylide that participates in the transmetallation step. An example Sonogashira reaction and the mechanism is shown below. This is a very useful reaction and an important addition to our synthesis toolbox.
The previous three reactions, Stille, Suzuki, and Sonogashira, share a similar mechanism that includes oxidative addition, transmetallation, and reductive elimination steps. Other reactions with different metals have a similar catalytic cycle with palladium. Although we won't discuss them here, you are well equipped to understand these reactions when you see them in the literature. Two of the most common examples are the Negishi reaction (transmetallation with zinc) and the Kumada reaction (transmetallation with magnesium).
Heck Reaction
The Heck reaction proceeds via a different mechanistic pathway than the Stille, Suzuki, or Sonogashira reactions because it does not contain transmetallation or reductive elimination steps. Instead, the Heck reaction relies on an alkene insertion step for carbon-carbon bond formation and beta hydrogen elimination to generate the product. (As a reminder, these steps were described in detail in the previous section.) An example of the reaction and its mechanism are shown below. A few key points about the mechanism are worth highlighting. The alkene insertion step usually places the Pd at the more substituted position and the carbon at the least substituted position. This step is a syn addition to the alkene which necessitates a bond rotation in the next step to place a beta hydrogen syn to the Pd. There are two possible Hs that can end up syn but the conformation that places the phenyl and the ketone anti is preferred. (Note that for this mechanism, only one beta carbon has Hs. The other beta carbon is the ketone. In many reactions, there are several alkenes that can form. Heck reactions generally produce the most stable possible alkene product.) This conformation yields the more stable trans alkene product in the beta hydrogen elimination step. The Pd(0) catalyst is regenerated in the final deprotonation step that explains the need for base in the mechanism.

Heck reactions are commonly run intermolecularly, between two different reactants. However, the true power of the Heck reaction for synthesis becomes apparent when studying intramolecular Heck reactions. This is a very useful technique for quickly building molecular complexity. We will see an example of this in one of the problems below.
Exercise \(\PageIndex{6}\)
Predict the product of the following intermolecular Heck reaction.
- Answer
-
This is a tricky problem. Most students quickly predict the conjugated diene shown below that doesn't form. This is a good illustration of the importance of evaluating potential products formed from the beta hydrogen elimination step. In this reaction, there are two beta carbons with Hs that can be eliminated with Pd. One option, the beta H on the left, yields the conjugated diene. The other option, the beta H on the right, yields an enol product that tautomerizes to the observed aldehyde product. Carbonyls are more stable than alkenes, so this thermodynamic difference drives the reaction to the aldehyde.
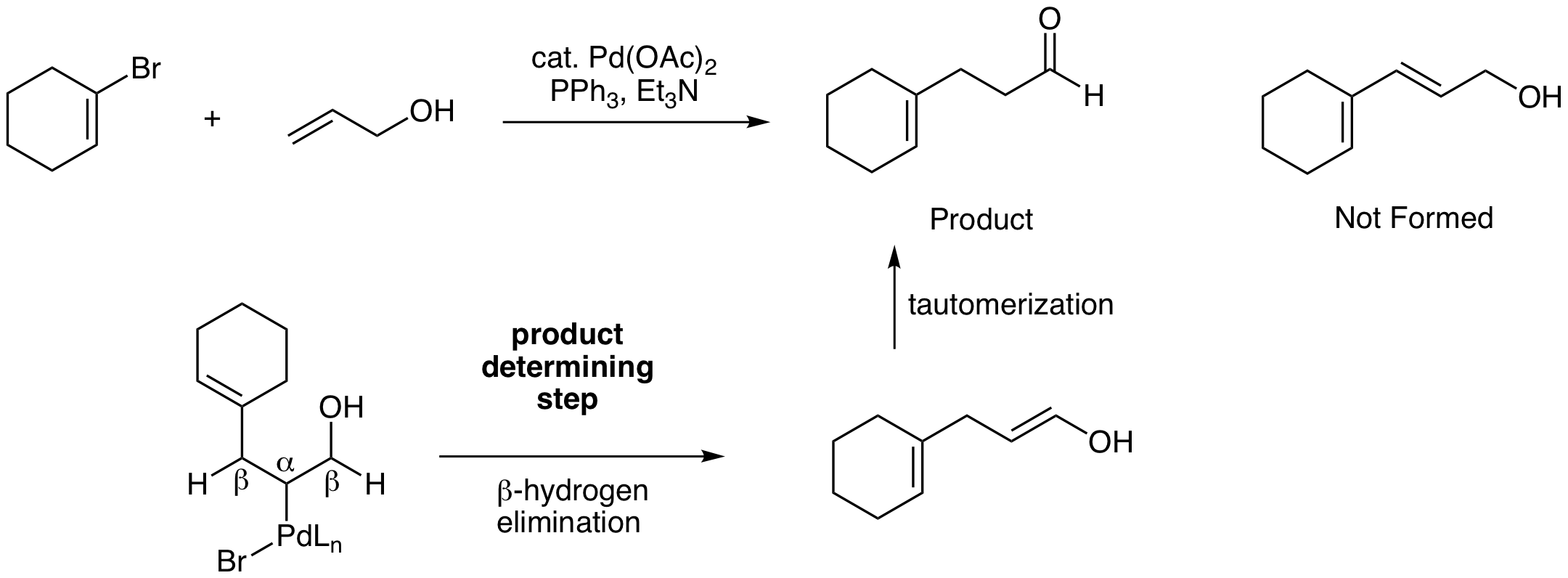
Exercise \(\PageIndex{7}\)
Predict the product of the following intramolecular Heck reaction.
- Answer
-
This is an excellent example of what is known as a Heck zipper reaction where multiple alkene insertions occur to form more than one ring. Working through the mechanism, we can see what is happening. After the initial oxidative addition step, the first alkene insertion occurs to form the new 6-membered ring. Normally, we would next perform a beta hydrogen elimination reaction to generate the product. However, this molecule has a quaternary carbon at the beta position, so beta hydrogen elimination is impossible. When that happens, the molecule undergoes another alkene insertion to form a second new ring. In this case, a new five membered ring. We again look to find a cis beta hydrogen. That is now possible when the Pd is on carbon #11 and the final product is formed.
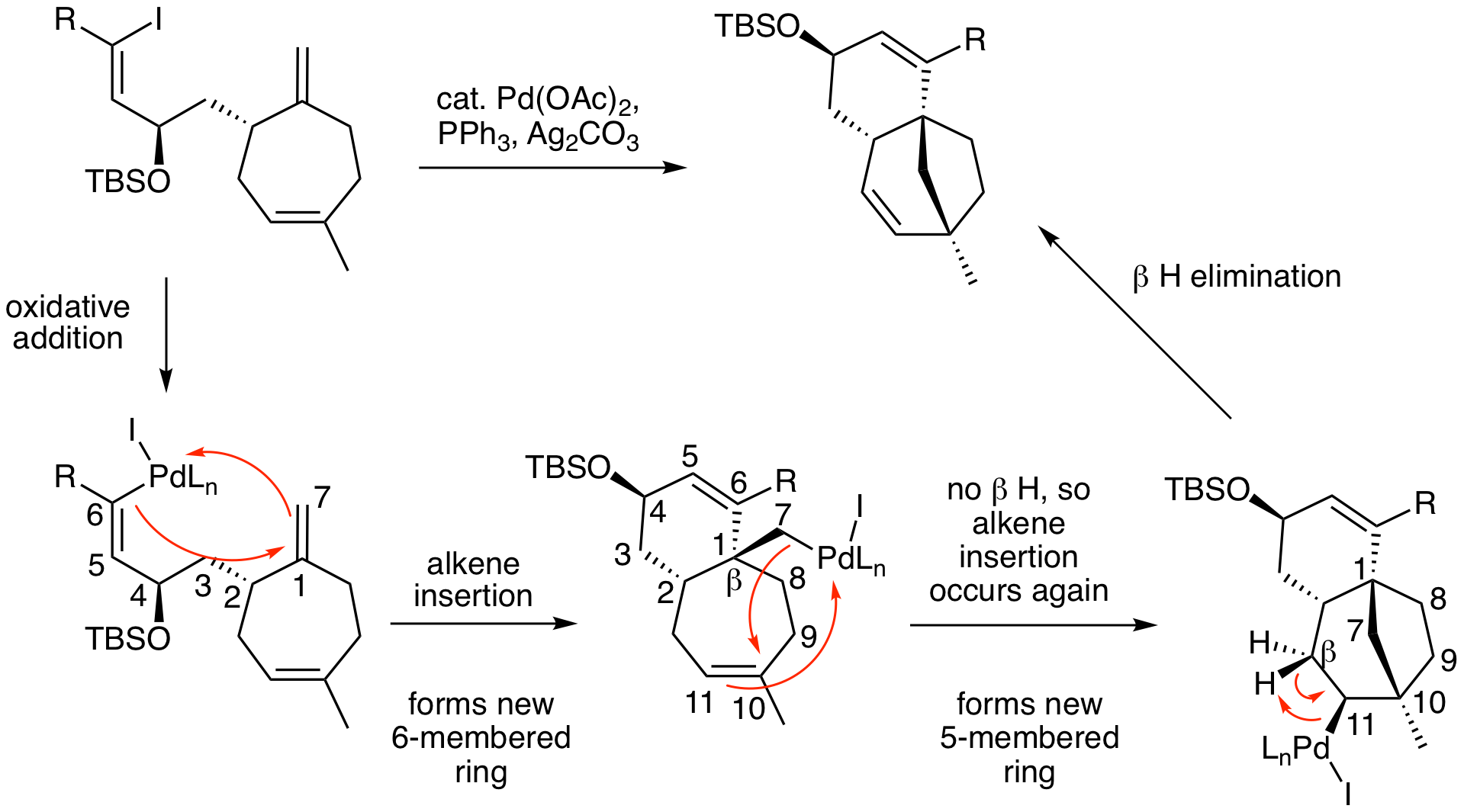
Contributors
- Prof. Kevin Shea (Smith College)

