16.S: Alkynes - An Introduction to Organic Synthesis (Summary)
- Page ID
- 453405
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Concepts & Vocabulary
9.1 Naming Alkynes (reference only)
- Follow IUPAC rules in naming alkynes.
9.2 Preparation of Alkynes - Elimination Reactions of Dihalides (reference only)
- Vicinal describes two groups on adjacent carbon atoms.
- Geminal describes two groups on the same carbon atom.
- Alkynes can be prepared by two successive eliminations of HX from either vicinal or geminal dihalides.
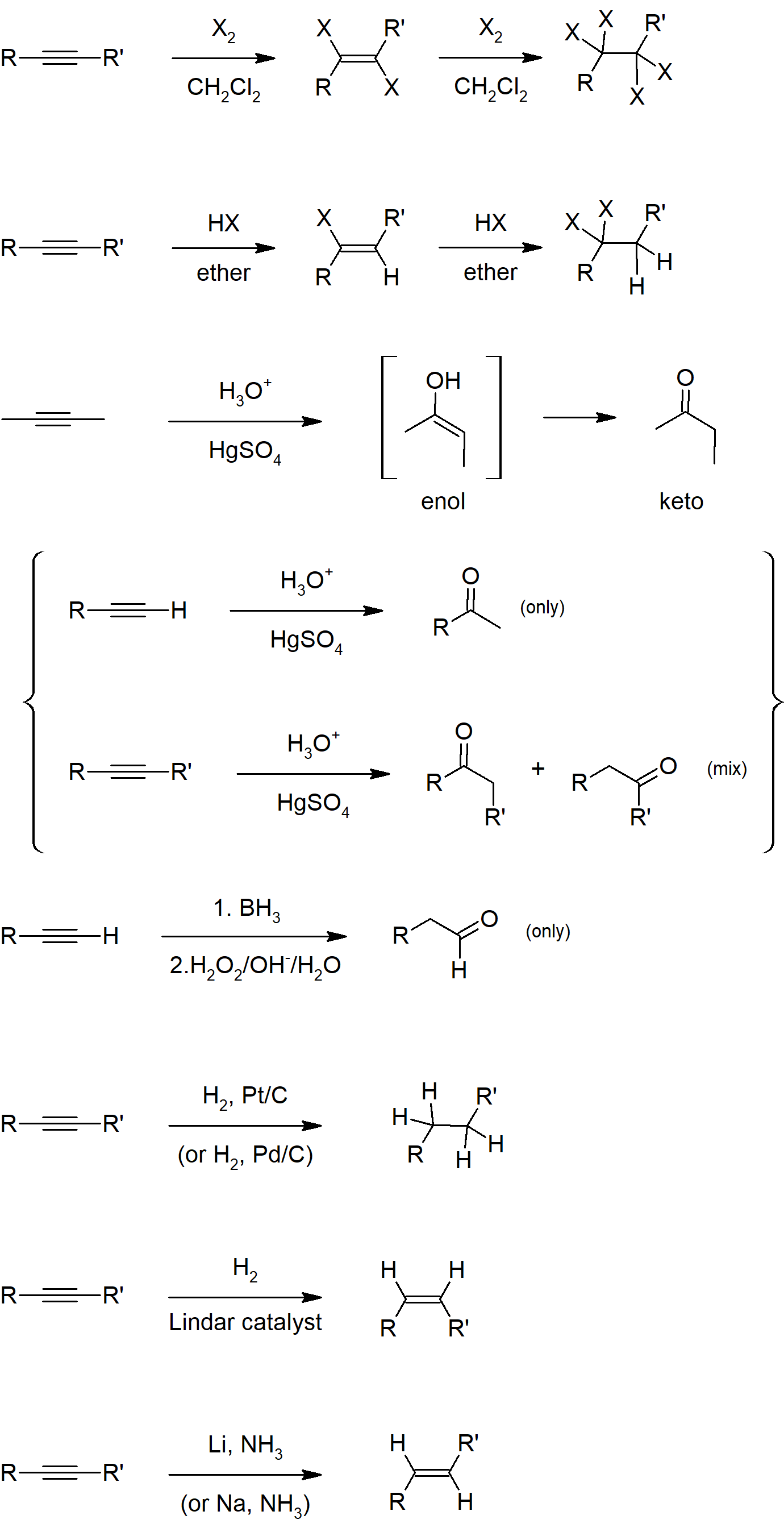
9.3 Reactions of Alkynes - Addition of HX and X2
- Alkynes undergo addition reactions similarly to alkenes yielding Markovnikov products.
- Enols have a hydroxyl group bonded to a sp2 hybrid carbon (double-bonded carbon).
- Enols are usually not stable and undergo keto-enol tautomerization to form a ketone or aldehyde.
- Hydration of alkynes leads to an enol product which then rapidly tautomerizes into a ketone or aldehyde.
- Alkynes can be hydrogenated with hydrogen gas and strong catalysts to yield alkanes.
- Alkynes can be hydrogenated with hydrogen gas and Lindlar's catalyst to yield Z alkenes.
- Alkynes can be hydrogenated with sodium metal and liquid ammonia to yield E alkenes.
9.6 Oxidative Cleavage of Alkynes (CHM 223)
- Oxidative cleavage of internal alkynes forms two molecules of carboxylic acids.
- Oxidative cleavage of terminal alkynes forms one molecule of carbon dioxide and one carboxylic acid.
9.7 Alkyne Acidity - Formation of Acetylide Anions (CHM 223)
- Terminal alkynes are relatively acidic compared to alkene and alkane carbon-hydrogen bonds.
- Deprotonation of a terminal alkyne forms an acetylide ion, which is a good nucleophile.
9.8 Alkylation of Acetylide Anions (CHM 223)
- Acetylide ions can be alkylated by adding to alkyl halides and carbonyl compounds.
9.9 An Introduction to Organic Synthesis (CHM 223)
- Desired products cannot always be made from available starting materials through one reaction. Formation of these materials may require multiple reactions completed in sequence. This type of reaction sequence is termed synthesis.
Skills to Master
- Skill 16.1 Draw addition mechanisms to alkynes incorporating carbocation intermediates.
- Skill 16.2 Draw addition mechanisms to alkynes incorporating halonium intermediates.
- Skill 16.3 Describe relative stability of enols to ketones and aldehydes.
- Skill 16.4 Draw keto-enol tautomerism mechanism.
- Skill 16.5 Draw products that differentiate between multiple reduction reactions of alkynes.
Summary of Reactions
Reactions of Alkynes