2.5: Naming Cycloalkanes
- Page ID
- 178963
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- name a substituted or unsubstituted cycloalkane, given its Kekulé structure, shorthand structure or condensed structure.
- draw the Kekulé, shorthand or condensed structure for a substituted or unsubstituted cycloalkane, given its IUPAC name.
Key Terms
Make certain that you can define, and use in context, the key terms below.
- cycloalkane
Study Notes
Provided that you have mastered the IUPAC system for naming alkanes, you should find that the nomenclature of cycloalkanes does not present any particular difficulties.
Many organic compounds found in nature contain rings of carbon atoms. These compounds are known as cycloalkanes. Cycloalkanes have one or more rings of carbon atoms. Cycloalkanes only contain carbon-hydrogen bonds and carbon-carbon single bonds. The simplest examples of this class consist of a single, un-substituted carbon ring, and these form a homologous series similar to the unbranched alkanes.
Like alkanes, cycloalkane molecules are often drawn as skeletal structures in which each intersection between two lines is assumed to have a carbon atom with its corresponding number of hydrogens. Cyclohexane, one of the most common cycloalkanes is shown below as an example.
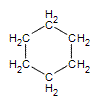.gif?revision=1&size=bestfit&width=85&height=92) same as
same as  same as
same as 
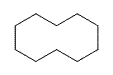
Cyclic hydrocarbons have the prefix "cyclo-". The IUPAC names, molecular formulas, and skeleton structures of the first ten cycloalkanes are given in Table 4.1.1. Note that the general formula for a cycloalkane composed of n carbons is CnH2n, and not CnH2n+2 as for alkanes. Although a cycloalkane has two fewer hydrogens than the equivalent alkane, each carbon is bonded to four other atoms so are still considered to be saturated with hydrogen.
| Table 4.1.1: Examples of Simple Cycloalkanes | ||
|---|---|---|
| Cycloalkane | Molecular Formula | Skeleton Structure |
| Cyclopropane | C3H6 |  |
| Cyclobutane | C4H8 |  |
| Cyclopentane | C5H10 |  |
| Cyclohexane | C6H12 |  |
| Cycloheptane | C7H14 | 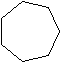 |
| Cyclooctane | C8H16 | 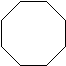 |
| Cyclononane | C9H18 | 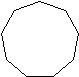 |
| Cyclodecane | C10H20 | 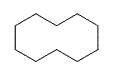 |
IUPAC Rules for Nomenclature
The naming of substituted cycloalkanes follows the same basic steps used in naming alkanes.
- Determine the parent chain.
- Number the substituents of the ring so that the sum of the numbers is the lowest possible.
- Name the substituents and place them in alphabetical order.
More specific rules for naming substituted cycloalkanes with examples are given below.
- Determine the cycloalkane to use as the parent. If there is an alkyl straight chain that has a greater number of carbons than the cycloalkane, then the alkyl chain must be used as the primary parent chain. Cycloalkanes substituents have an ending "-yl". If there are two cycloalkanes in the molecule, use the cycloalkane with the higher number of carbons as the parent.
Example 4.1.1
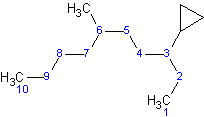
The longest straight chain contains 10 carbons, compared with cyclopropane, which only contains 3 carbons. The parent chain in this molecule is decane and cyclopropane is a substituent, . The name of this molecule is 3-cyclopropyl-3,10-dimethyldecane.
2) When there is only one substituent on the ring, the ring carbon attached to the substituent is automatically carbon #1. Indicating the number of the carbon with the substituent in the name is optional.
Example 4.1.2
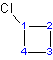 |
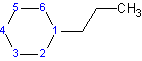 |
| 1-chlorocyclobutane or cholorocyclobutane | 1-propylcyclohexane or propylcyclohexane |
If there are multiple substituents on the ring, number the carbons of the cycloalkane so that the carbons with substituents have the lowest possible number. A carbon with multiple substituents should have a lower number than a carbon with only one substituent or functional group. One way to make sure that the lowest number possible is assigned is to number the carbons so that when the numbers corresponding to the substituents are added, their sum is the lowest possible.
Example 4.1.3
.gif?revision=1&size=bestfit&width=171&height=129) (1+3=4) NOT
(1+3=4) NOT .gif?revision=1&size=bestfit&width=171&height=132) (1+5=6)
(1+5=6)
3) When naming the cycloalkane, the substituents must be placed in alphabetical order. Remember the prefixes di-, tri-, etc. , are not used for alphabetization.
Example 4.1.4
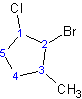
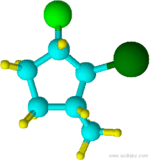
2-bromo-1-chloro-3-methylcyclopentane
Notice that "f" of fluoro alphabetically precedes the "m" of methyl.
Example 4.1.5
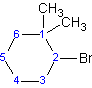
(2-bromo-1,1-dimethylcyclohexane)
Although "di" alphabetically precedes "f", "di" is not used in determining the alphabetical order.
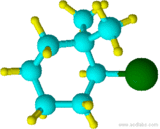
Example 4.1.6

(2-fluoro-1,1,-dimethylcyclohexane NOT 1,1-dimethyl-2-fluorocyclohexane)
Hydrocarbons having more than one ring are common, and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of n carbon atoms associated with m rings the formula is: CnH(2n + 2 - 2m). The structural relationship of rings in a polycyclic compound can vary. They may be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following table.
| Isolated Rings | Spiro Rings | Fused Rings | Bridged Rings |
|---|---|---|---|
| No common atoms | One common atom | One common bond | Two common atoms |
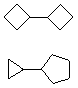 |
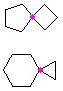 |
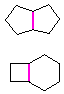 |
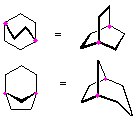 |
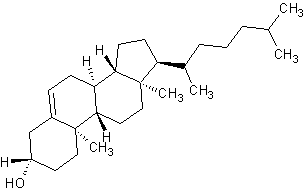
Polycyclic compounds, like cholesterol shown below, are biologically important and typically have common names accepted by IUPAC. However, the common names do not generally follow the basic IUPAC nomenclature rules, and will not be covered here.
|
|
|
Cholesterol (polycyclic) |
Problems
Name the following structures. (Note: The structures are complex for practice purposes and may not be found in nature.)
1)  2)
2) 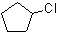 3)
3)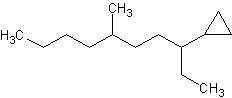 4)
4)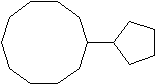 5)
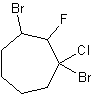
5)
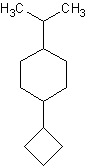
6)
Draw the following structures.
7) 1,1-dibromo-3-butyl-5-fluoro-7-methylcyclooctane
8) 1,1-dibromo-2,3-dichloro-4-propylcyclobutane
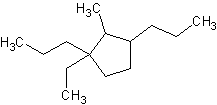
9) 1-ethyl-2-methyl-1,3-dipropylcyclopentane
Name the following structures.
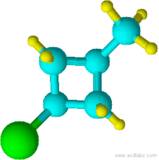
Blue=Carbon Yellow=Hydrogen Green=Chlorine
10).gif?revision=1&size=bestfit&width=102&height=105) 11)
11)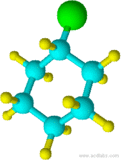 12)
12)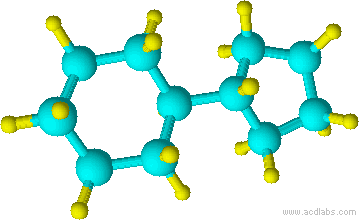 13)
13)
Answers to Practice Problems
1) cyclodecane 2) chlorocyclopentane or 1-chlorocyclopentane
3) 6-methyl-3-cyclopropyldecane 4) cyclopentylcyclodecane or 1-cyclopentylcyclodecane 5) 1,3-dibromo-1-chloro-2-fluorocycloheptane
6) 1-cyclobutyl-4-isopropylcyclohexane
7)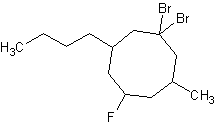 8)
8)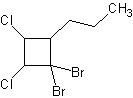 9)
9)
10) cyclohexane 11) chlorocyclohexane 12) cyclopentylcyclohexane 13) 1-chloro-3-methylcyclobutane
Outside links
- More Practice Problems on Nomenclature of Cycloalkanes
- Vollhardt, Schore. Organic Chemistry. 5th ed.
- Wikipedia: Cycloalkanes
- http://www.cem.msu.edu/~reusch/VirtualText/nomen1.htm
- http://www.chemguide.co.uk/organicprops/alkanes/background.html
- http://www.cem.msu.edu/~reusch/VirtualText/nomen1.htm
- http://science.csustan.edu/nhuy/chem...IVNamecyal.htm
- http://en.wikibooks.org/wiki/Organic...s/Cycloalkanes
References
- ACD/ChemSketch Freeware, version 11.0, Advanced Chemistry Development, Inc., Toronto, ON, Canada, www.acdlabs.com, 2008.
- Bruice, Paula Yurkanis. Oragnic Chemistry. 5th. CA. Prentice Hall, 2006.
- Fryhle, C.B. and G. Solomons. Organic Chemistry. 9th ed. Danvers, MA: Wiley, 2008.
- McMurry, John. Organic Chemistry. 7th ed. Belmont, California: Thomson Higher Education, 2008.
- Sadava, Heller, Orians, Purves, Hillis. Life The Science of Biology. 8th ed. Sunderland, MA: W.H. Freeman, 2008.
- Vollhardt, K. Peter C., and Neil E. Schore. Organic Chemistry. 5th ed. New York: W.H. Freeman, 2007.
Exercises
Contributors
- Pwint Zin
- Jim Clark (ChemGuide)
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
- Dr. Kelly Matthews (Senior Professor of Chemistry, Harrisburg Area Community College)