3.2: Drying Agents
- Page ID
- 490682
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Drying Agents
Why They are Used
An organic layer is always treated with a drying agent after having been exposed to water in a separatory funnel (step c) in Table 4.4). Drying agents are anhydrous inorganic materials that favorably form "hydrates", which incorporate water molecules into their solid lattice structure (for example, \(\ce{Na_2SO_4} \cdot 7 \ce{H_2O}\)). A drying agent is swirled with an organic solution to remove trace amounts of water.
Many organic solvents dissolve a significant portion of water (Table 4.6) that must be removed before rotary evaporation, or else water will be found in the concentrated product. After solvent removal using a rotary evaporator, it occasionally happens that so much water is present that droplets or a second layer is seen amongst the oily liquid in a round-bottomed flask. The presence of water with the product makes the yield inaccurate, and water also must be removed before GC-MS analysis, as water is incompatible with mass-spectrometer detectors.
| Solvent | Grams water dissolved in 100 mL solvent |
|---|---|
| Diethyl ether | 1.24 g |
| Ethyl acetate | 2.92 g |
| Dichloromethane (\(CH_2Cl_2\)) | 0.32 g |
| Hexanes | 0.007 g |
Drying agents must be used with even relatively nonpolar organic solvents that do not theoretically dissolve much water, as water may cling to the sides of the separatory funnel and inadvertently travel with the organic layer while draining. Additionally, solutes dissolved in an organic layer with polar functional groups (e.g. alcohols, carboxylic acids) can hydrogen-bond with water and increase the likelihood of water dissolving in the organic layer.
Types of Drying Agents

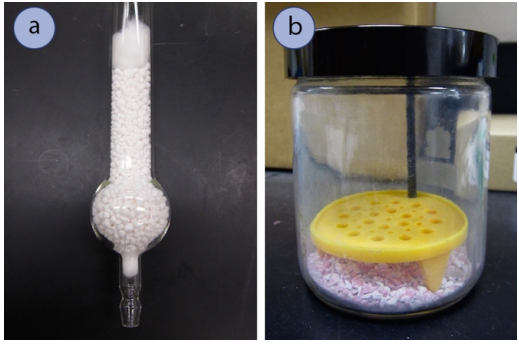
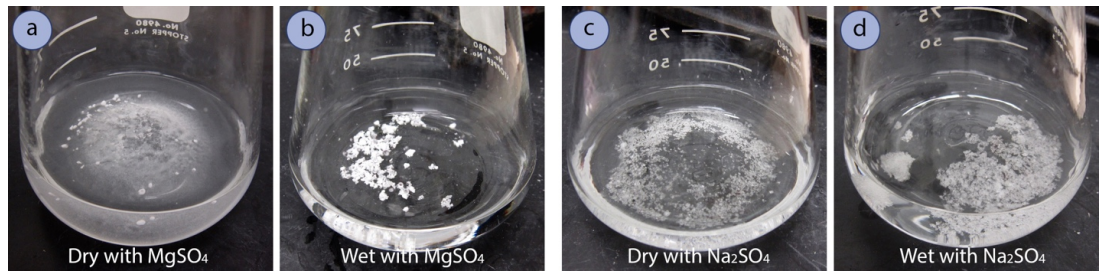
Drying agents (Figure 4.48) remove trace amounts of water from organic solutions by forming hydrates. The most useful drying agents indicate when they have completely absorbed all of the water from the solution. Anhydrous magnesium sulfate \(\left( \ce{MgSO_4} \right)\) is a fine, loose powder (Figure 4.49a), but its hydrate is clumpy and often clings to the glass (Figure 4.49b). A typical drying procedure is to add anhydrous \(\ce{MgSO_4}\) to an organic solution until it stops clumping and fine particles are seen, which indicate that there is no longer water available to form the clumpy hydrates.

Anhydrous calcium sulfate \(\left( \ce{CaSO_4} \right)\), can be purchased containing a cobalt compound that is blue when dry and pink when wet (this is then sold under the name Drierite, Figure 4.49c+d). In this way, blue Drierite can be used as a visual indicator for the presence of water.\(^8\)
The most common drying agents used to remove water from organic solutions are anhydrous sodium sulfate \(\left( \ce{Na_2SO_4} \right)\) and anhydrous magnesium sulfate \(\left( \ce{MgSO_4} \right)\). Many chemists consider \(\ce{MgSO_4}\) the "go-to" drying agent as it works quickly, holds a lot of water for its mass, and the hydrates are noticeably chunkier compared to the anhydrous form, making it easy to see when you've added enough. A drawback to using \(\ce{MgSO_4}\) is that it is a fine powder, and so the solutions must be subsequently filtered to remove the drying agent. Another drawback to \(\ce{MgSO_4}\) is that all fine powders heavily adsorb product on their surface (which is why they must be rinsed with solvent after filtration), and sometimes more granular drying agents are used to minimize the loss of product by adsorption.
In some procedures \(\ce{Na_2SO_4}\) or \(\ce{CaCl_2}\) are used if they seem to work just as well as \(\ce{MgSO_4}\), or if the solution is incompatible with \(\ce{MgSO_4}\) (see Table 4.8). A procedural advantage to these drying agents is that their granules are not easily dispersed, allowing for the solutions to be easily decanted (poured). In many situations drying agents are interchangeable (see Table 4.8 for a survey of drying agents). However, it is most common for desiccators and drying tubes to use \(\ce{CaSO_4}\) or \(\ce{CaCl_2}\) (Figure 4.50), as they can be easily manipulated in their pellet or rock forms.
| Drying Agent | Hydrate formula(s) | Practical Comments | Other Comments |
|---|---|---|---|
| Magnesium sulfate | \(\ce{MgSO_4} \cdot 7 \ce{H_2O}\) | Quickly removes most water, and can hold a lot for its mass (\(0.15\)-\(0.75 \: \text{g}\) water per \(\text{g}\) desiccant).\(^9\) Is a fine powder, so must be gravity filtered. Its high surface area means it will somewhat adsorb compound: be sure to rinse after filtering. | \(\ce{Mg(H_2O)_4^{2+}}\) is somewhat acidic, so is incompatible with highly acid-sensitive groups. |
| Sodium Sulfate |
\(\ce{Na_2SO_4} \cdot 7 \ce{H_2O}\) \(\ce{Na_2SO_4} \cdot 10 \ce{H_2O}\) |
Removes water at a moderate rate, so the solution should be allowed to sit with the drying agent for some time. Can hold a lot of water for its mass (\(1.25 \: \text{g}\) water per \(\text{g}\) desiccant), but may leave small amounts of water remaining. Solutions with \(\ce{Na_2SO_4}\) can usually be decanted. | Cannot dry diethyl ether well unless a brine wash was used. |
| Calcium chloride |
\(\ce{CaCl_2} \cdot 2 \ce{H_2O}\) \(\ce{CaCl_2} \cdot 6 \ce{H_2O}\) |
Quickly removes water well, although larger quantities are needed than other drying agents (holds \(0.30 \: \text{g}\) water per \(\text{g}\) desiccant). If using a fine powder, the solution must be gravity filtered and drying agent rinsed. If using pellets, the solution should be allowed to sit for a few minutes, then decanted. | Absorbs water as well as methanol and ethanol. |
| Calcium sulfate (Drierite) |
\(\ce{CaSO_4} \cdot \frac{1}{2} \ce{H_2O}\) \(\ce{CaSO_4} \cdot 2 \ce{H_2O}\) |
Quickly removes water, but needs large quantities as it holds little water per gram. Are most often used in desiccators and drying tubes, not with solutions. |
Drying Agents Procedure
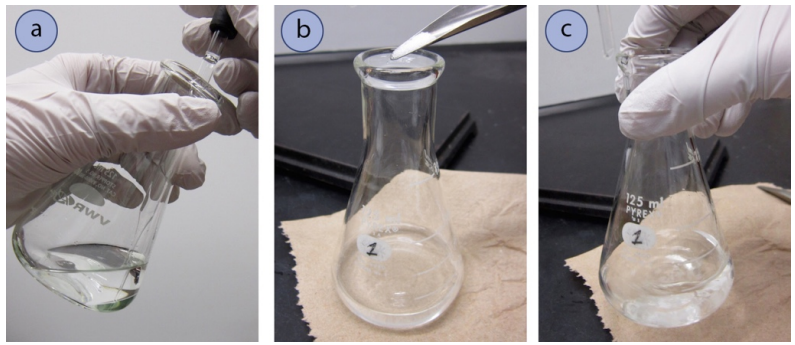
- The organic solution to be dried must be in an Erlenmeyer flask, as solutions can easily splash out of beakers when swirled.
- First inspect the solution to see if it's homogenous, or if there is a second layer of liquid (typically a puddle on the bottom). If a second layer is noticed, this is probably water and the majority of it should be pipetted out before continuing on (Figure 4.51a). It can be difficult to completely remove a water layer by pipette, so leaving a tiny bit is acceptable.
- Add a small portion of drying agent to the flask,the size of one pea for macroscale work (Figure 4.51b), and swirl the solution (Figure 4.51c). Be sure to close the jar of drying agent when not in use, as the reagents are hygroscopic. After a short period of time, inspect the mixture closely.
- If the entire drying agent clumps into pieces that are much larger than the original size (Figure 4.52b+c), there is still water remaining in the flask. Add another portion of drying agent and swirl.
- A solution is nearing dryness when fine particles are noticed that don't cling to other particles (Figure 4.52a+c) or to the glass when swirled (Figure 4.53a). A wet organic solution can be cloudy, and a dry one is always clear.
- If using anhydrous \(\ce{Na_2SO_4}\), allow the solution to sit for at least 5 minutes before declaring the solution dry, as this reagent takes time to work.
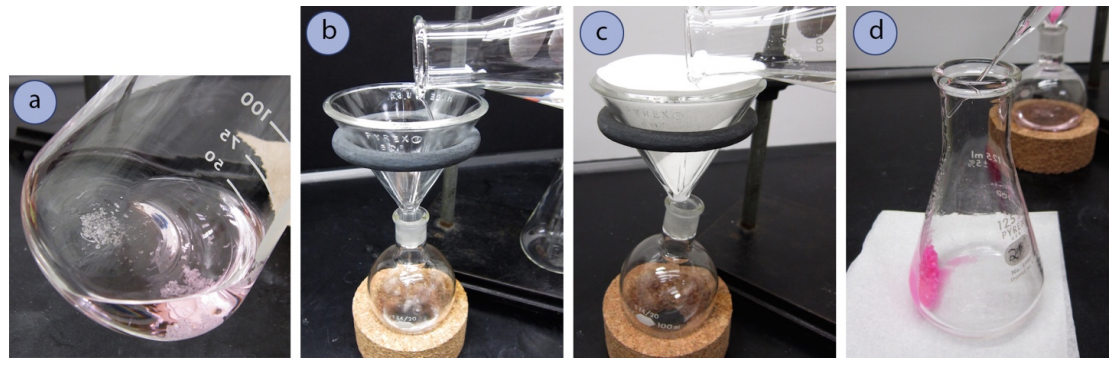
- When the solution is dry, separate the drying agent from the solution:
- If using \(\ce{Na_2SO_4}\), \(\ce{CaCl_2}\) pellets, or \(\ce{CaSO_4}\) rocks, carefully decant the solution into an appropriately sized round-bottomed flask (Figure 4.53b), being sure to fill the flask no more than halfway. Reminder: a mass of the empty flask should be obtained if the solvent will be evaporated on the rotary evaporator.
- If using \(\ce{MgSO_4}\), gravity filter the solution into an appropriately sized round-bottomed flask (Figure 4.53c). When pouring, leave the solid behind as long as possible (essentially decant the solution, but into the funnel lined with filter paper). Solid can slow drainage in the filter paper.
- With all drying agents, rinse the drying agent (in the flask and in the filter funnel) with a few \(\text{mL}\) of fresh organic solvent, and add the rinsing to the round-bottomed flask (Figure 4.53d). Remove the solvent using a rotary evaporator.
\(^4\)A. Seidell, Solubilities of Inorganic and Organic Substances, D. Van Nostrand Company, 1907.
\(^5\)When assessing the result of a litmus paper test, look at the center of the drop. The center is the most concentrated spot, and it's possible a color change may not be seen on the outside where the solution has spread and diluted. Any pink seen on blue litmus paper means the solution is acidic.
\(^6\)From: Fessenden, Fessenden, Feist, Organic Laboratory Techniques, 3\(^\text{th}\) ed., Brooks-Cole, 2001.
\(^7\)From: Fessenden, Fessenden, Feist, Organic Laboratory Techniques, 3\(^\text{th}\) ed., Brooks-Cole, 2001.
\(^8\)Blue Drierite is expensive, so is commonly used by mixing it together with white Drierite (\(\ce{CaSO_4}\) without the cobalt indicator). Pink (wet) Drierite can be dried by spreading it on a watch glass and drying in a \(110^\text{o} \text{C}\) oven overnight.
\(^9\)Grams water per gram of desiccant values are from: J. A. Dean, Lange's Handbook of Chemistry, 15\(^\text{th}\) ed., McGraw-Hill, 1999, Sect. 11.2. \(\ce{CaCl_2}\) value is quoted for the formation of \(\ce{CaCl_2} \cdot 2 \ce{H_2O}\).


