2.5: Arrangement of Electron (Shell Model)
- Page ID
- 15564
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)An electron shell is the outside part of an atom around the atomic nucleus. It is a group of atomic orbitals with the same value of the principal quantum number \(n\). Electron shells have one or more electron subshells, or sublevels. The name for electron shells comes from the Bohr model, in which groups of electrons were believed to go around the nucleus at certain distances, so that their orbits formed "shells".
Introduction
What more could we want to know about the structure of an atom? We know that atoms contain positively and negatively charged particles, and that the number of these charges in each atom is different for each element. We also know that the positive charges are concentrated in a tiny nucleus, and that the electrons move around the nucleus in a space that is much, much larger than the nucleus.
However, some of the most important questions we asked in the previous Concept Development Study are still unanswered. Remember that we saw that carbon and nitrogen have very similar atomic masses. Now we can add that these elements have very similar atomic numbers, so their atoms have similar numbers of protons and electrons. But carbon and nitrogen are, in most chemical and physical ways, very different. Similarly, some elements like sodium and potassium have very different atomic numbers but have quite similar chemical and physical properties. It seems that comparing the properties of two different atoms is not very easy to understand just from comparing the numbers of protons and electrons the atoms contain.
To continue to understand the answers to these questions, we need even more detail about the structure of each type of atom. Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together.
Shells
An electron shell may be thought of as an orbit followed by electrons around an atom nucleus. Because each shell can contain only a fixed number of electrons, each shell is associated with a particular range of electron energy, and thus each shell must fill completely before electrons can be added to an outer shell. The electrons in the outermost shell determine the chemical properties of the atom (see Valence shell). For an explanation of why electrons exist in these shells see electron configuration.
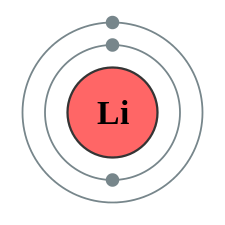
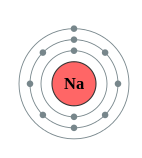
Figure \(\PageIndex{1}\): A shell Diagram of lithium (left) and Sodium (right)
The electron shells are labeled K, L, M, N, O, P, and Q; or 1, 2, 3, 4, 5, 6, and 7; going from innermost shell outwards. Electrons in outer shells have higher average energy and travel farther from the nucleus than those in inner shells. This makes them more important in determining how the atom reacts chemically and behaves as a conductor, because the pull of the atom's nucleus upon them is weaker and more easily broken. In this way, a given element's reactivity is highly dependent upon its electronic configuration.
Valence Electrons
The valence shell is the outermost shell of an atom in its uncombined state, which contains the electrons most likely to account for the nature of any reactions involving the atom and of the bonding interactions it has with other atoms. Valence electrons are electrons that are associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron can only be in the outermost electron shell.
An atom with a closed shell of valence electrons tends to be chemically inert. An atom with one or two valence electrons more than a closed shell is highly reactive, because the extra valence electrons are easily removed to form a positive ion. An atom with one or two valence electrons fewer than a closed shell is also highly reactive, because of a tendency either to gain the missing valence electrons (thereby forming a negative ion), or to share valence electrons (thereby forming a covalent bond).
Like an electron in an inner shell, a valence electron has the ability to absorb or release energy in the form of a photon. An energy gain can trigger an electron to move (jump) to an outer shell; this is known as atomic excitation. Or the electron can even break free from its associated atom's valence shell; this is ionization to form a positive ion. When an electron loses energy (thereby causing a photon to be emitted), then it can move to an inner shell which is not fully occupied.
The number of valence electrons of an element can be determined by the periodic table group (vertical column) in which the element is categorized. With the exception of groups 3–12 (the transition metals), the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column.
| Periodic table group | Valence electrons |
|---|---|
| Group 1 (I) (alkali metals) | 1 |
| Group 2 (II) (alkaline earth metals) | 2 |
| Groups 3-12 (transition metals) | 2* |
| Group 13 (III) (boron group) | 3 |
| Group 14 (IV) (carbon group) | 4 |
| Group 15 (V) (pnictogens) | 5 |
| Group 16 (VI) (chalcogens) | 6 |
| Group 17 (VII) (halogens) | 7 |
| Group 18 (VIII or 0) (noble gases) | 8** |
* The general method for counting valence electrons is generally not useful for transition metals.
** Except for helium, which has only two valence electrons.
Contributors
- Connections (John Hutchinson)
- Wikipedia

