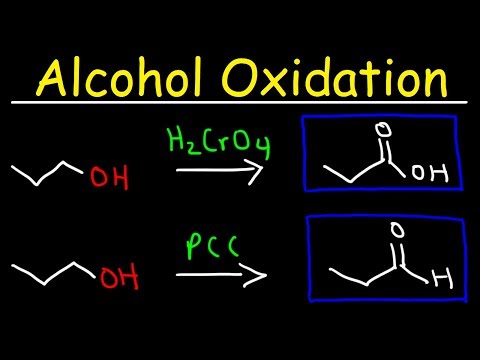
19.6: Oxidation of alcohols and aldehydes
- Page ID
- 225885
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Oxidizing agents
The oxidation of an alcohol to form an aldehyde or ketone is very important in synthesis. In this process, the hydroxy hydrogen of the alcohol is replaced by a leaving group (X in the figure below).
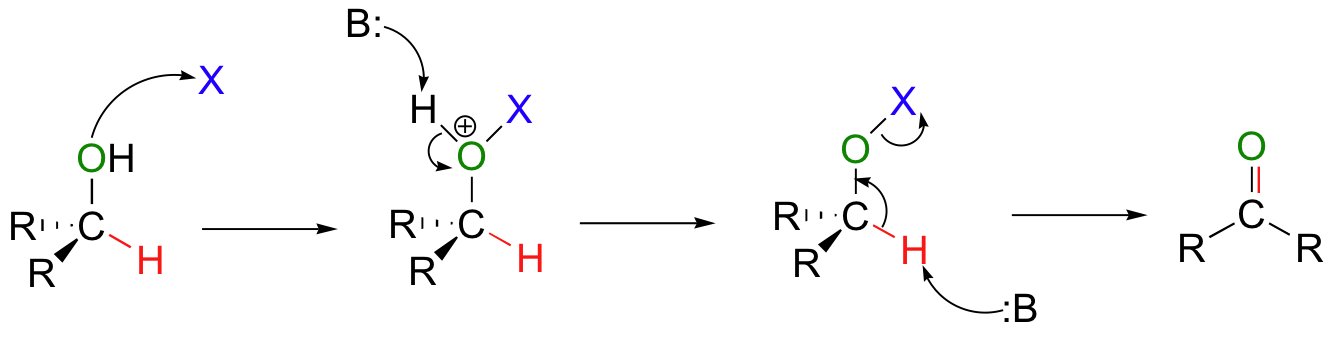
Then, a base can abstract the proton bound to the alcohol carbon, which results in elimination of the X leaving group and formation of a new carbon-oxygen double bond. As you can see by looking closely at this general mechanism, tertiary alcohols cannot be oxidized in this way – there is no hydrogen to abstract in the final step!
Oxidation using chromic acid
A common method for oxidizing secondary alcohols to ketones uses chromic acid (H2CrO4) as the oxidizing agent. Chromic acid, also known as Jones reagent, is prepared by adding chromium trioxide (CrO3) to aqueous sulfuric acid. The Jones oxidation also uses acetone as a co-solvent in the reaction to prevent over-oxidation of the organic product.
A mechanism for the chromic acid oxidation of a ketone is shown below.
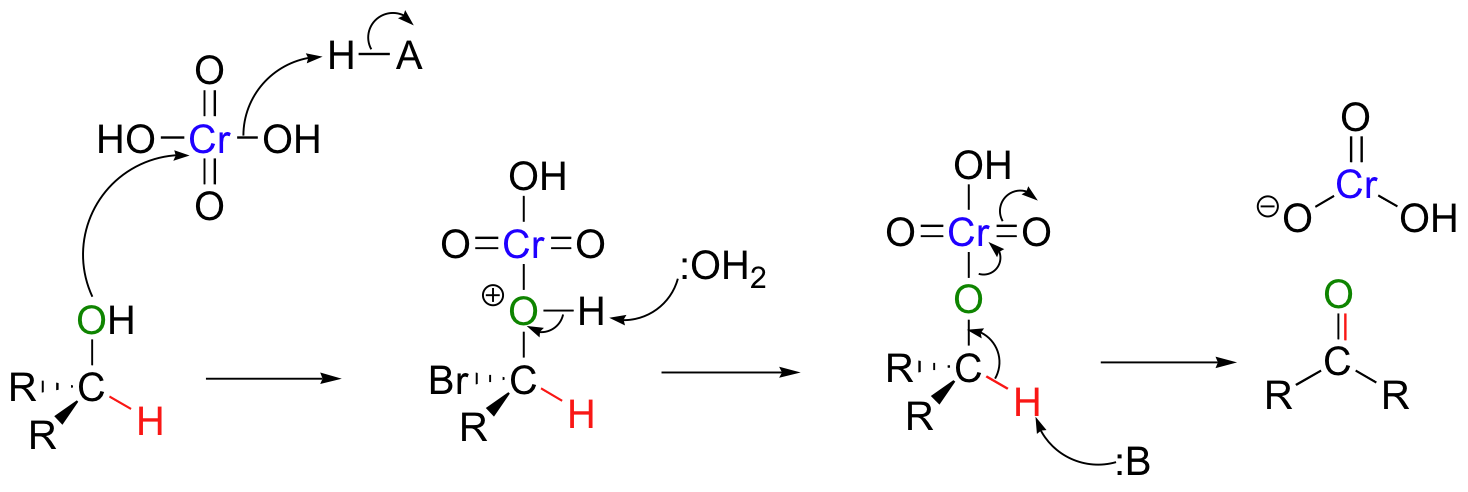
Note that the chromium reagent has lost two bonds to oxygen in this reaction, and thus has been reduced (it must have been reduced – it is the oxidizing agent!).
Ketones are not oxidized by chromic acid, so the reaction stops at the ketone stage. In contrast, primary alcohols are oxidized by chromic acid first to aldehydes, then straight on to carboxylic acids.
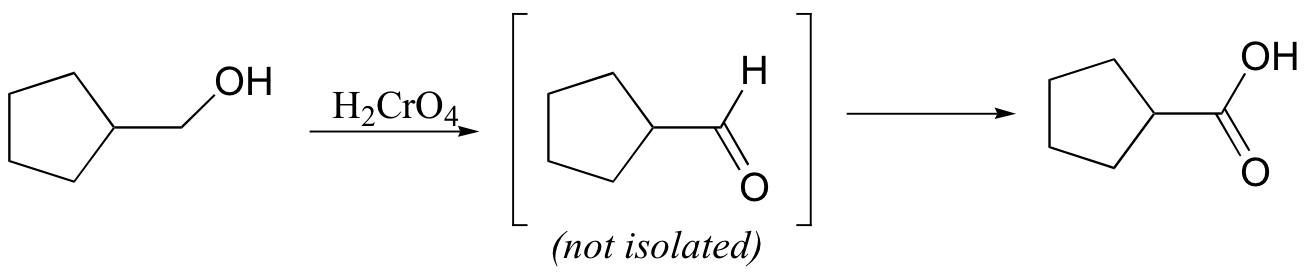
It is actually the hydrate form of the aldehyde that is oxidized:
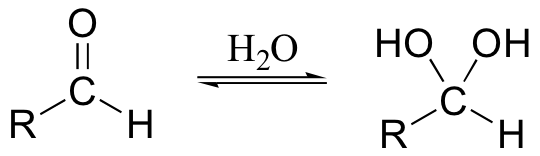
One of the hydroxyl groups of the hydrate attacks chromic acid, and the reaction proceeds essentially as shown for the oxidation of a secondary alcohol.
Under some conditions, chromic acid will even oxidize a carbon in the benzylic position to a carboxylic acid (notice that a carbon-carbon bond is broken in this transformation). We saw this reaction using KMnO4 in section 16.3; either chromic acid or KMnO4 is suitable, and they give the same carboxylic acid product.
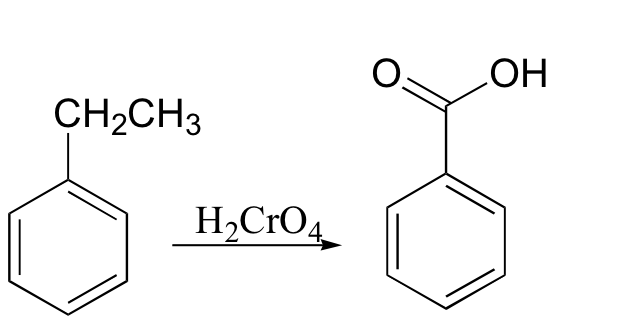
A number of other common oxidizing agents are discussed below.
The related chromium(VI) compound pyridinium chlorochromate (PCC) is also useful for oxidizing primary alcohols to aldehydes. Further oxidation of the aldehyde to the carboxylic acid stage does not occur, because the reaction is carried out in anhydrous (water-free) organic solvents such as dichloromethane, and therefore the hydrate form of the aldehyde is not able to form.
Pyridinium chlorochromate is generated by combining chromium trioxide, hydrochloric acid, and pyridine.
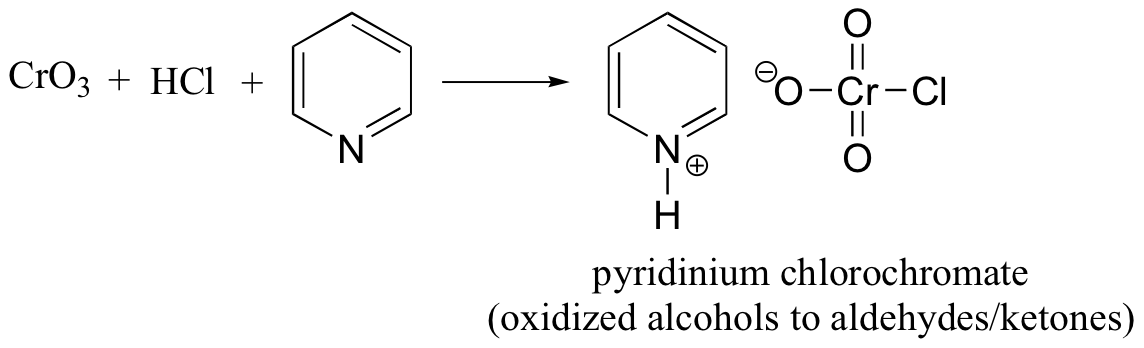
The PCC oxidation conditions can both also be used to oxidize secondary alcohols to ketones.
Swern oxidation
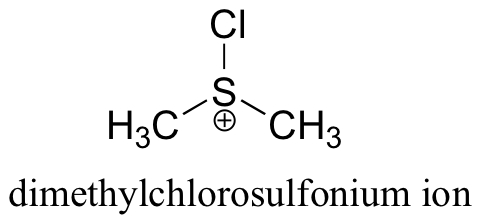
The Swern oxidation uses dimethylsulfoxide and oxalyl chloride, followed by addition of a base such as triethylamine. The actual oxidizing species in this reaction is the dimethylchlorosulfonium ion, which forms from dimethylsulfoxide and oxalyl chloride.
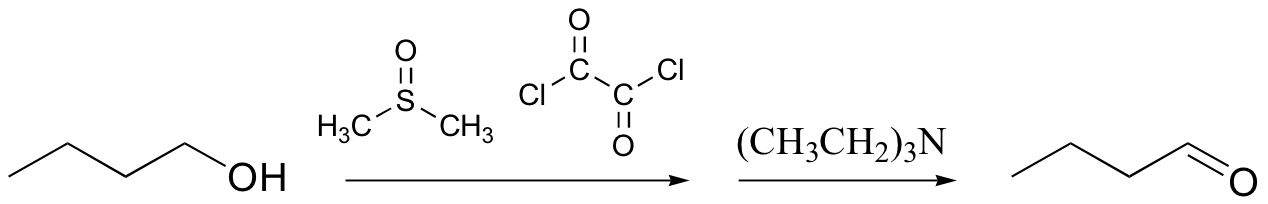
The mechanism is included below, for reference:
Oxidation-Swern Oxidation (Stage1)
 In the first stage dimethylsulfoxide (DMSO) reacts with oxalyl chloride to give an electrophilic sulfur compound, which collapses to give a chlorosulfonium ion, CO2 and CO.
In the first stage dimethylsulfoxide (DMSO) reacts with oxalyl chloride to give an electrophilic sulfur compound, which collapses to give a chlorosulfonium ion, CO2 and CO. Oxidation-Swern Oxidation (Stage2)
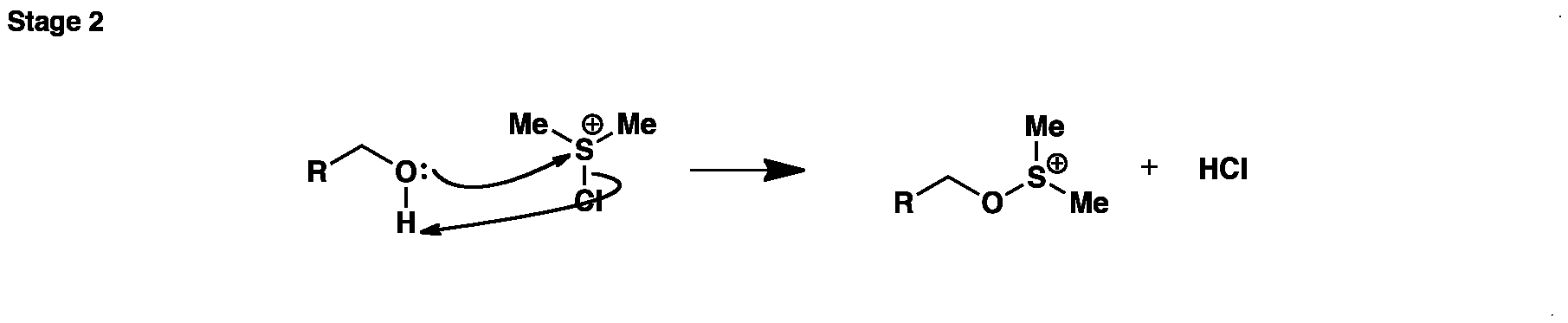 In the second stage the alchohol reacts with the chlorosulfonium ion to give a new sulfonium salt and HCl.
In the second stage the alchohol reacts with the chlorosulfonium ion to give a new sulfonium salt and HCl. Oxidation-Swern Oxidation (Stage3)
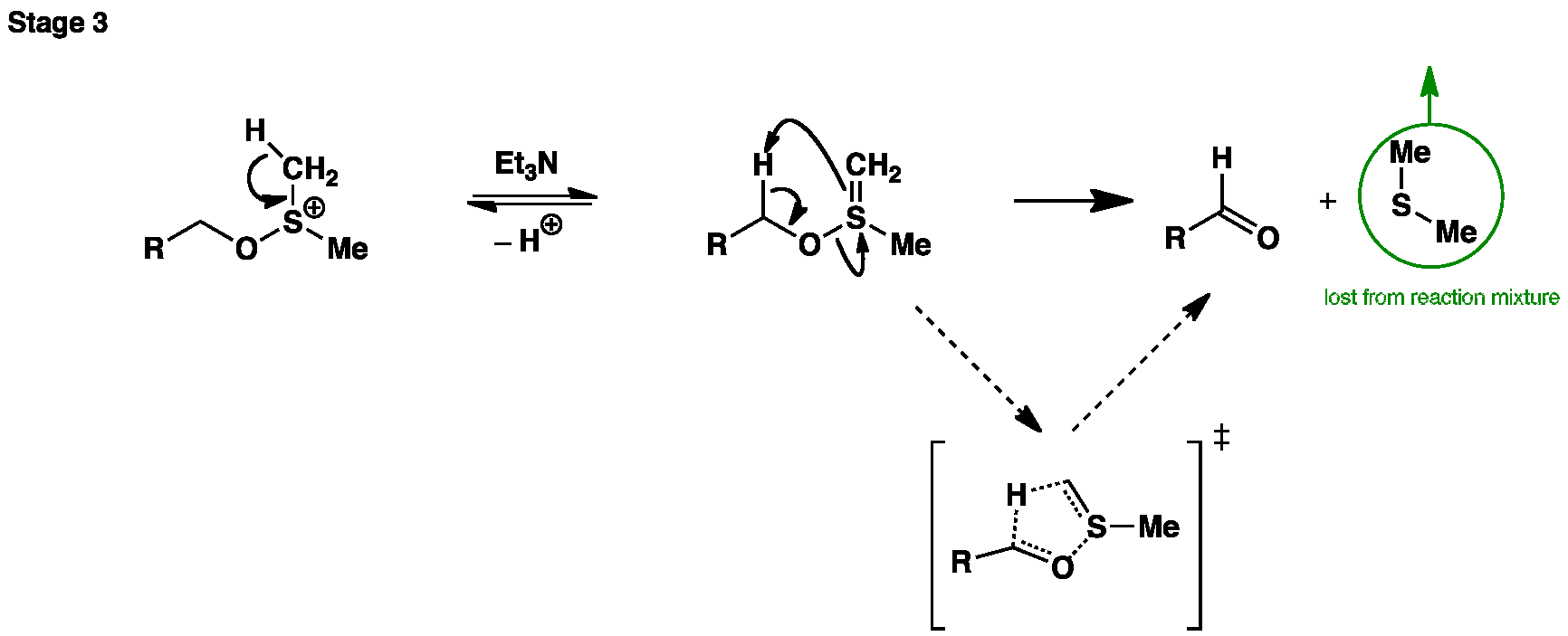 In the final stage the base (Et3N) is added to deprotonate the salt, which then collapses to give the desired aldehyde or ketone and dimethyl sulfide.
In the final stage the base (Et3N) is added to deprotonate the salt, which then collapses to give the desired aldehyde or ketone and dimethyl sulfide.Oxidation using silver(I)
Silver ion, Ag(I), is often used to oxidize aldehydes to ketones. Two common reaction conditions are:
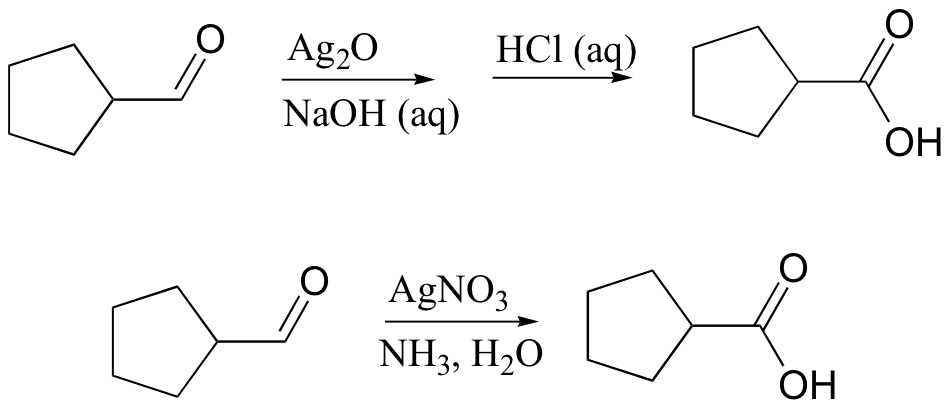
The set of reagents in the latter reaction conditions are commonly known as ‘Tollens’ reagent’.
Video

- Organic Chemistry With a Biological Emphasis . Authored by: Tim Soderberg. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg). Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Oxidation of Aldehydes and Ketones. Authored by: Dr. Dietmar Kennepohl, Prof. Steven Farmer, Jim Clark. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_19%3A_Aldehydes_and_Ketones%3A_Nucleophilic_Addition_Reactions/19.03_Oxidation_of_Aldehydes_and_Ketones. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Oxidation-Swern Oxidation Stages 1-3. Authored by: Nick Greeves. Located at: https://chem.libretexts.org/Under_Construction/ChemTube3D/Organic_Reactions/Oxidation-Swern_Oxidation_(Stage1). Project: Chemistry LibreText. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike