4.6: Separating enantiomers
- Page ID
- 225783
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- describe a common process for separating a mixture of enantiomers.
- explain why racemic mixtures do not rotate plane-polarized light.
Key TERMS
Make certain that you can define, and use in context, the key terms below.
- racemic mixture (or racemate)
- resolve
Study Notes
A racemic mixture is a 50:50 mixture of two enantiomers. Because they are mirror images, each enantiomer rotates plane-polarized light in an equal but opposite direction and is optically inactive. If the enantiomers are separated, the mixture is said to have been resolved. A common experiment in the laboratory component of introductory organic chemistry involves the resolution of a racemic mixture.
The dramatic biochemical consequences of chirality are illustrated by the use, in the 1950s, of the drug Thalidomide, a sedative given to pregnant women to relieve morning sickness. It was later realized that while the (+)‑form of the molecule, was a safe and effective sedative, the (−)‑form was an active teratogen. The drug caused numerous birth abnormalities when taken in the early stages of pregnancy because it contained a mixture of the two forms.
As noted earlier, chiral compounds synthesized from achiral starting materials and reagents are generally racemic (i.e. a 50:50 mixture of enantiomers). Separation of racemates into their component enantiomers is a process called resolution. Since enantiomers have identical physical properties, such as solubility and melting point, resolution is extremely difficult. Diastereomers, on the other hand, have different physical properties, and this fact is used to achieve resolution of racemates. Reaction of a racemate with an enantiomerically pure chiral reagent gives a mixture of diastereomers, which can be separated. For example, if a racemic mixture of a chiral alcohol is reacted with a enantiomerically pure carboxylic acid, the result is a mixture of diastereomers: in this case, because the pure (R) enantiomer of the acid was used, the product is a mixture of (R-R) and (R-S) diastereomeric esters, which can, in theory, be separated by their different physical properties. Subsequent hydrolysis of each separated ester will yield the ‘resolved’ (enantiomerically pure) alcohols.
As noted earlier, chiral compounds synthesized from achiral starting materials and reagents are generally racemic (i.e. a 50:50 mixture of enantiomers). Separation of racemates into their component enantiomers is a process called resolution. Since enantiomers have identical physical properties, such as solubility and melting point, resolution is extremely difficult. Diastereomers, on the other hand, have different physical properties, and this fact is used to achieve resolution of racemates. Reaction of a racemate with an enantiomerically pure chiral reagent gives a mixture of diastereomers, which can be separated. Reversing the first reaction then leads to the separated enantiomers plus the recovered reagent.
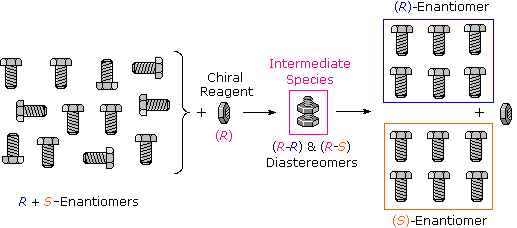
Many kinds of chemical and physical reactions, including salt formation, may be used to achieve the diastereomeric intermediates needed for separation. The above figure illustrates this general principle by showing how a nut having a right-handed thread (R) could serve as a “reagent” to discriminate and separate a mixture of right- and left-handed bolts of identical size and weight. Only the two right-handed partners can interact to give a fully-threaded intermediate, so separation is fairly simple. The resolving moiety, i.e. the nut, is then removed, leaving the bolts separated into their right and left-handed forms. Chemical reactions of enantiomers are normally not so dramatically different, but a practical distinction is nevertheless possible.
Because the physical properties of enantiomers are identical, they seldom can be separated by simple physical methods, such as fractional crystallization or distillation. It is only under the influence of another chiral substance that enantiomers behave differently, and almost all methods of resolution of enantiomers are based upon this fact. We include here a discussion of the primary methods of resolution.
Chiral amines as resolving agents and resolution of racemic acids
The most commonly used procedure for separating enantiomers is to convert them to a mixture of diastereomers that will have different physical properties: melting point, boiling point, solubility, and so on. For example, if you have a racemic or D,L mixture of enantiomers of an acid and convert this to a salt with a chiral base having the D configuration, the salt will be a mixture of two diastereomers, (D acid . D base) and (L acid . D base). These diastereomeric salts are not identical and they are not mirror images. Therefore they will differ to some degree in their physical properties, and a separation by physical methods, such as crystallization, may be possible. If the diastereomeric salts can be completely separated, the acid regenerated from each salt will be either exclusively the D or the L enantiomer:

Resolution of chiral acids through the formation of diastereomeric salts requires adequate supplies of suitable chiral bases. Brucine, strychnine, and quinine frequently are used for this purpose because they are readily available, naturally occurring chiral bases. Simpler amines of synthetic origin, such as 2-amino- 1 -butanol, amphetamine, and 1 -phenylethanamine, also can be used, but first they must be resolved themselves.
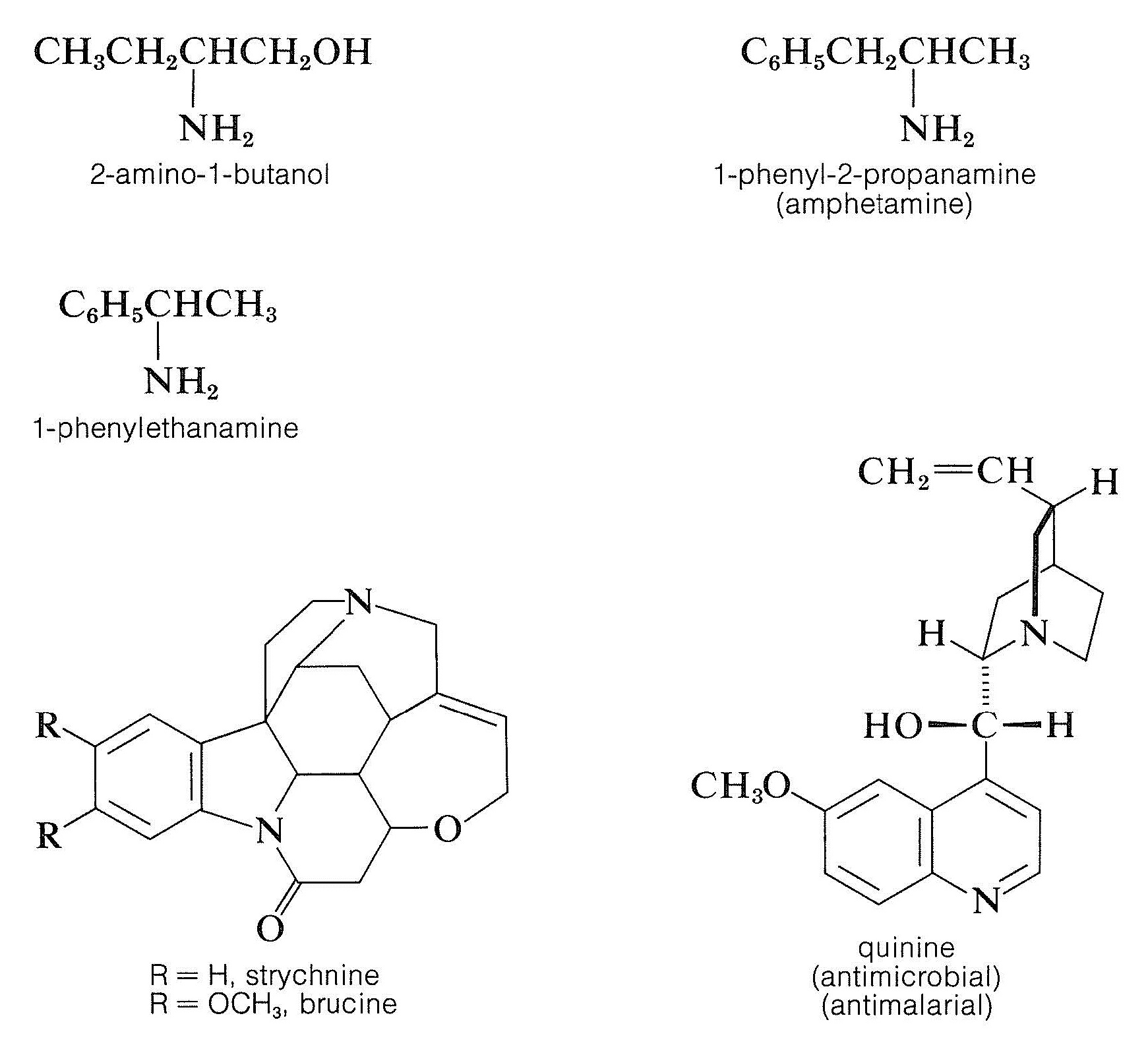
Resolution of racemic bases
Chiral acids, such as (+)-tartaric acid, (-)-malic acid, (-)-mandelic acid, and (+)-camphor- 10-sulfonic acid, are used for the resolution of a racemic base.

The principle is the same as for the resolution of a racemic acid with a chiral base, and the choice of acid will depend both on the ease of separation of the diastereomeric salts and, of course, on the availability of the acid for the scale of the resolution involved. Resolution methods of this kind can be tedious, because numerous recrystallizations in different solvents may be necessary to progressively enrich the crystals in the less-soluble diastereomer. To determine when the resolution is complete, the mixture of diastereomers is recrystallized until there is no further change in the measured optical rotation of the crystals. At this stage it is hoped that the crystalline salt is a pure diastereomer from which one pure enantiomer can be recovered. The optical rotation of this
enantiomer will be a maximum value if it is “optically” pure because any amount of the other enantiomer could only reduce the magnitude of the measured rotation $$\alpha$$.
Resolution of racemic alcohols
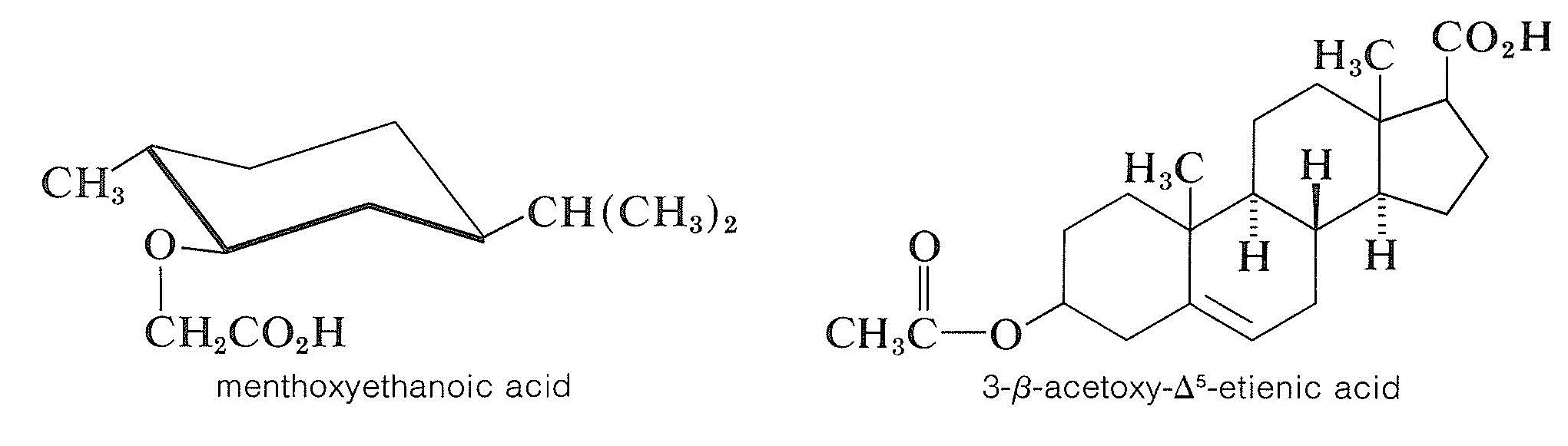
To resolve a racemic alcohol, a chiral acid can be used to convert the alcohol to a mixture of diastereomeric esters. This is not as generally useful as might be thought because esters tend to be liquids unless they are very high-molecularweight compounds. If the diastereomeric esters are not crystalline, they must be separated by some other method than fractional crystallization (for instance, by chromatography methods, Section 9-2). Two chiral acids that are useful resolving agents for alcohols are:
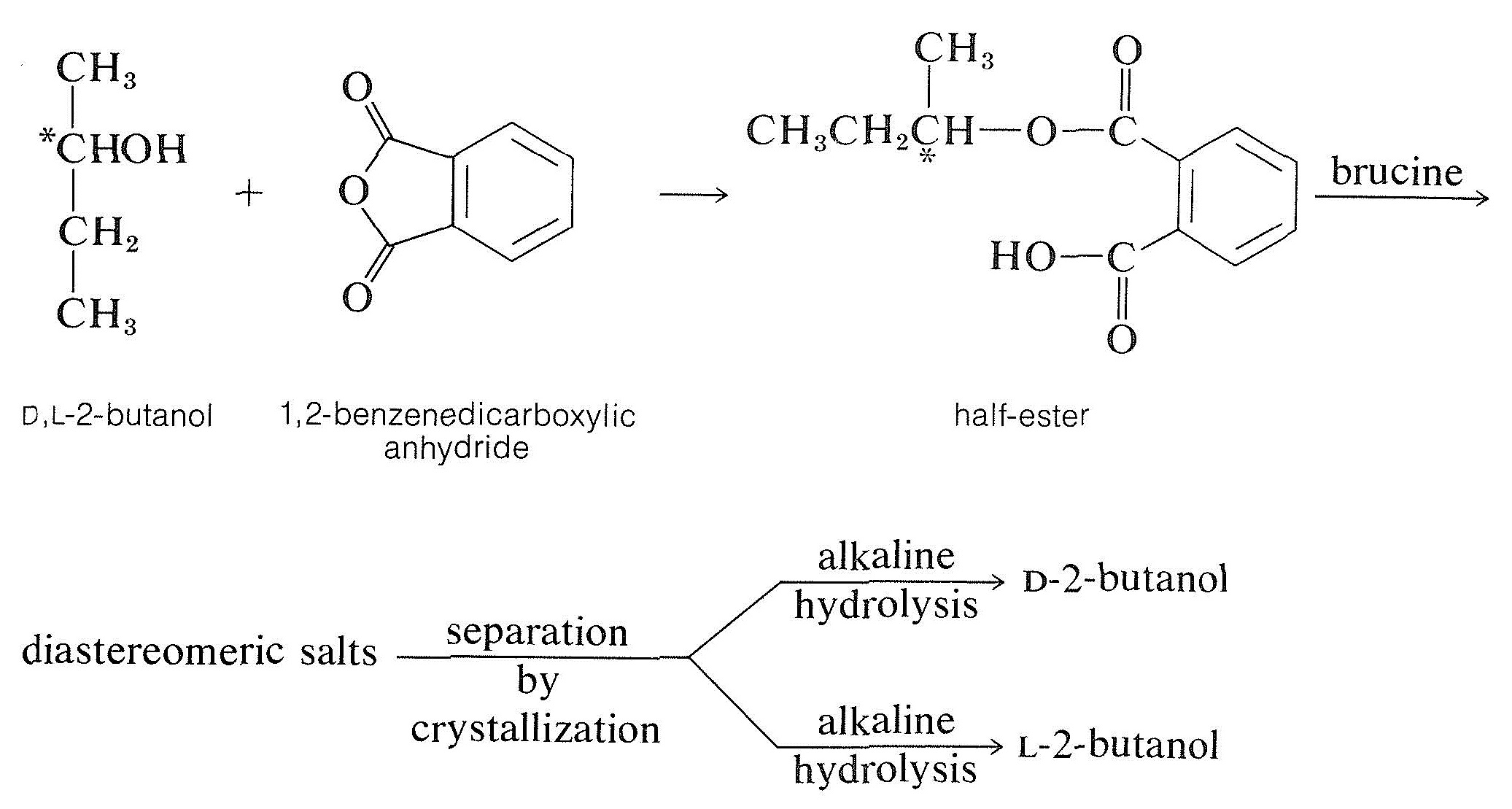
The most common method of resolving an alcohol is to convert it to a half-ester of a dicarboxylic acid, such as butanedioic (succinic) or 1,2-benzenedicarboxylic (phthalic) acid, with the corresponding anhydride. The resulting half-ester has a free carboxyl function and may then be resolvable with a chiral base, usually brucine:
Other methods of resolution
Qne of the major goals in the field of organic chemistry is the development of reagents with the property of “chiral recognition” such that they can effect a clean separation of enantiomers in one operation without destroying either of the enantiomers. We have not achieved that ideal yet, but it may not be far in the future. Chromatographic methods (related to TLC and GC), whereby the stationary phase is a chiral reagent that adsorbs one enantiomer more strongly than the other, have been used to resolve racemic compounds, but such resolutions seldom have led to both pure enantiomers on a preparative scale. Other methods, called kinetic resolutions, are excellent when applicable, taking advantage of differences in reaction rates of enantiomers with chiral reagents. One enantiomer may react more rapidly than the other, thereby leaving an excess of the other enantiomer behind. For example, racemic tartaric acid can be resolved with the aid of certain penicillin molds that consume the dextrorotatory enantiomer faster than the levorotatory enantiomer. As a result, almost pure (-)-tartaric acid can be recovered from the mixture:
(±)-tartaric acid + mold $$\rightarrow$$ (-)-tartaric acid + more mold
A disadvantage of resolutions of this type is that the more reactive enantiomer usually is not recoverable from the reaction mixture.
The crystallization procedure employed by Pasteur for his classical resolution of (±)-tartaric acid (see the history part of section 4.1.) has been successful only in a very few cases. This procedure depends on the formation of individual crystals of each enantiomer. Thus if the crystallization of sodium ammonium tartrate is carried out below 27″, the usual racemate salt does not form; a mixture of crystals of the (+) and (-) salts forms instead. The two different kinds of crystals, which are related as an object to its mirror image, can be separated manually with the aid of a microscope and subsequently may be converted to the tartaric acid enantiomers by strong acid. A variation on this method of resolution is the seeding of a saturated solution of a racemic mixture with crystals of one pure enantiomer in the hope of causing crystallization of just that one enantiomer, thereby leaving the other in solution. Unfortunately, very few practical resolutions have been achieved in this way.
Even when a successful resolution is achieved, some significant problems remain. For instance, the resolution itself does not provide information on the actual configuration of the (+) or (-) enantiomer. Also, it is not possible to tell the enantiomeric purity (optical purity) of the resolved enantiomers without additional information. This point is discussed further in the next section.
Exercise
Indicate the reagents you would use to resolve the following compounds. Show the reactions involved and specify the physical method you believe would be the best to separate the diastereomers.
- 1-phenyl-2-propanamine
- 2,3-pentadienedioic acid
- 1-phenylethanol
- Answer:
- [reveal-answer q=”816347″]Show Solution[/reveal-answer]
[hidden-answer a=”816347″] - a. React 1-phenyl-2-propanamine racemic mixture with a chiral acid such as (+)-tartaric acid (R, R).Reaction will produce a mixture of diastereomeric salts (i.e. R, R, R and S, R, R).Separate diastereomers through crystallization.
- Treat salt with strong base (e.g. KOH) to recover the pure enantiomeric amine.
- b. React 2,3-pentadienedioic acid mixture with a chiral base such as (R)‑1‑phenylethylamine.Reaction will produce a mixture of diastereomeric salts.Separate diastereomers through crystallization.Treat salt with strong acid (e.g. HCl) to recover the pure enantiomer acid.c. React 1-phenylethanol mixture with 1,2-benzenedicarboxylic anhydride.Reaction will produce a mixture of diastereomeric salts.Separate diastereomers through crystallization.Then alkaline hydrolysis treatment to recover the pure enantiomeric alcohol.
[/hidden-answer]
- Racemic Mixtures and the Resolution of Enantiomers. Authored by: Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University) Prof. Steven Farmer (Sonoma State University) John D. Robert and Marjorie C. Caserio (1977). Located at: https://chem.libretexts.org/LibreTexts/Athabasca_University/Chemistry_350%3A_Organic_Chemistry_I/Chapter_5%3A_Stereochemistry_at_Tetrahedral_Centres/5.08_Racemic_Mixtures_and_the_Resolution_of_Enantiomers. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike. License Terms: Content from Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format.

