18.5: Radical Polymerization of Alkenes, Polymers
- Page ID
- 225879
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Note
Many natural materials—such as proteins, cellulose and starch, and complex silicate minerals—are polymers. Artificial fibers, films, plastics, semisolid resins, and rubbers are also polymers. More than half the compounds produced by the chemical industry are synthetic polymers.
Chain-Reaction (Addition) Polymerization
The polymerization can be represented by the reaction of a few monomer units:
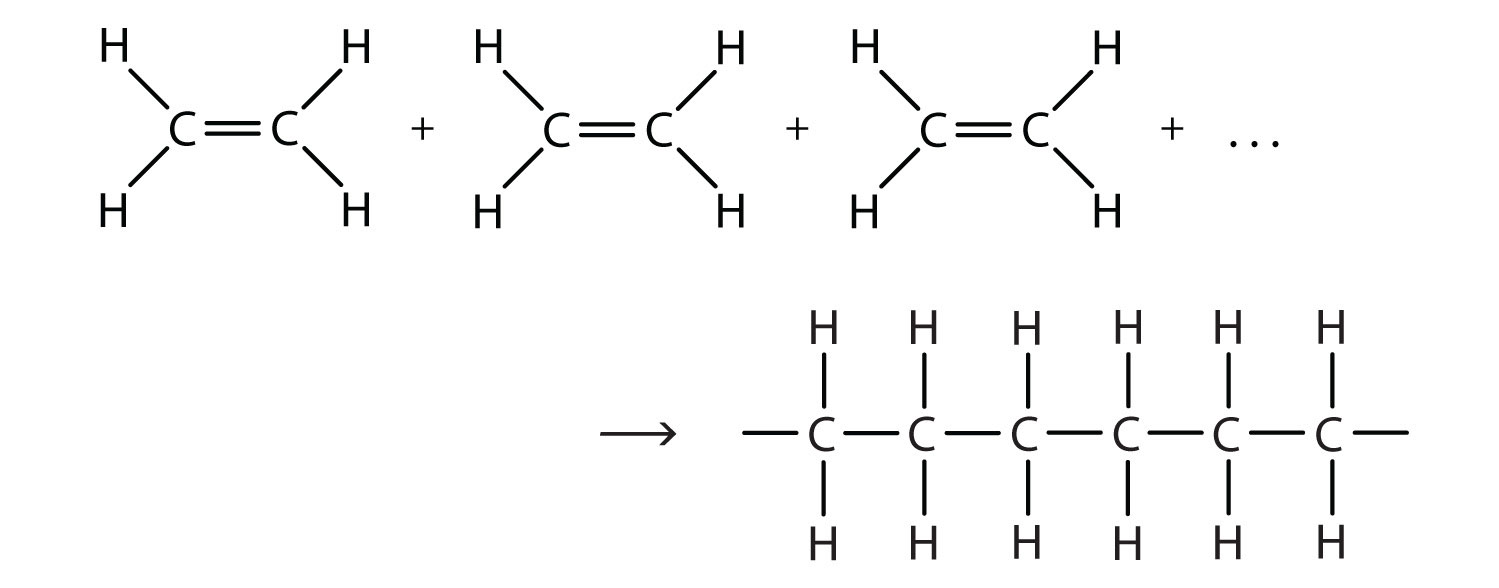
The bond lines extending at the ends in the formula of the product indicate that the structure extends for many units in each direction. Notice that all the atoms—two carbon atoms and four hydrogen atoms—of each monomer molecule are incorporated into the polymer structure. Because displays such as the one above are cumbersome, the polymerization is often abbreviated as follows:
nCH2=CH2 → [ CH2CH2 ] n
During the polymeriation of ethene, thousands of ethene molecules join together to make poly(ethene) – commonly called polythene. The reaction is done at high pressures in the presence of a trace of oxygen as an initiator.
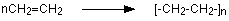
Some common addition polymers are listed in the Table below . Note that all the monomers have carbon-to-carbon double bonds. Many polymers are mundane (e.g., plastic bags, food wrap, toys, and tableware), but there are also polymers that conduct electricity, have amazing adhesive properties, or are stronger than steel but much lighter in weight.
| Monomer | Polymer | Polymer Name | Some Uses |
|---|---|---|---|
| CH2=CH2 | ~CH2CH2CH2CH2CH2CH2~ | polyethylene | plastic bags, bottles, toys, electrical insulation |
| CH2=CHCH3 | 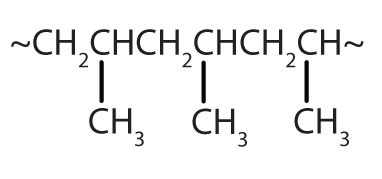 | polypropylene | carpeting, bottles, luggage, exercise clothing |
| CH2=CHCl | 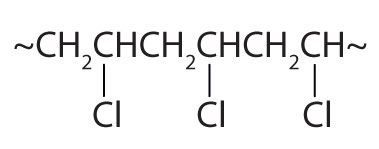 | polyvinyl chloride | bags for intravenous solutions, pipes, tubing, floor coverings |
| CF2=CF2 | ~CF2CF2CF2CF2CF2CF2~ | polytetrafluoroethylene | nonstick coatings, electrical insulation |
Step 1: Chain Initiation
The oxygen reacts with some of the ethene to give an organic peroxide. Organic peroxides are very reactive molecules containing oxygen-oxygen single bonds which are quite weak and which break easily to give free radicals. You can short-cut the process by adding other organic peroxides directly to the ethene instead of using oxygen if you want to. The type of the free radicals that start the reaction off vary depending on their source. For simplicity we give them a general formula: $$Ra ^{\bullet}$$
Step 2: Chain Propagation
In an ethene molecule, CH2=CH2, the two pairs of electrons which make up the double bond aren’t the same. One pair is held securely on the line between the two carbon nuclei in a sigma bond. The other pair is more loosely held in an orbital above and below the plane of the molecule in a $$\pi$$ bond.
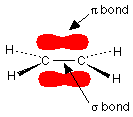
Note
It would be helpful – but not essential – if you read about the structure of ethene before you went on. If the diagram above is unfamiliar to you, then you certainly ought to read this background material.
Imagine what happens if a free radical approaches the $$\pi$$ bond in ethene.
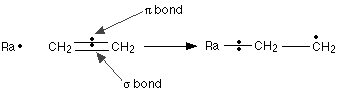
Note
Don’t worry that we’ve gone back to a simpler diagram. As long as you realise that the pair of electrons shown between the two carbon atoms is in a $$\pi$$ bond – and therefore vulnerable – that’s all that really matters for this mechanism.
The sigma bond between the carbon atoms isn’t affected by any of this. The free radical, Ra![]() , uses one of the electrons in the $$\pi$$ bond to help to form a new bond between itself and the left hand carbon atom – a radical addition step. The other electron returns to the right hand carbon. You can show this using curved arrow notation (with single-headed “fish-hook” arrows) if you want to:
, uses one of the electrons in the $$\pi$$ bond to help to form a new bond between itself and the left hand carbon atom – a radical addition step. The other electron returns to the right hand carbon. You can show this using curved arrow notation (with single-headed “fish-hook” arrows) if you want to:
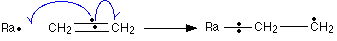
This is energetically worth doing because the new bond between the radical and the carbon is stronger than the $$\pi$$ bond which is broken. You would get more energy out when the new bond is made than was used to break the old one. The more energy that is given out, the more stable the system becomes. What we’ve now got is a bigger free radical – lengthened by CH 2 CH 2 . That can react with another ethene molecule in the same way:

So now the radical is even bigger. That can react with another ethene – and so on and so on. The polymer chain gets longer and longer.
Step 3: Chain Termination
The chain does not, however, grow indefinitely. Sooner or later two free radicals will collide together.
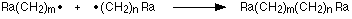
That immediately stops the growth of two chains and produces one of the final molecules in the poly(ethene). It is important to realise that the poly(ethene) is going to be a mixture of molecules of different sizes, made in this sort of random way. Because chain termination is a random process, poly(ethene) will be made up of chains of different lengths.
Step-Reaction (Condensation) Polymerization
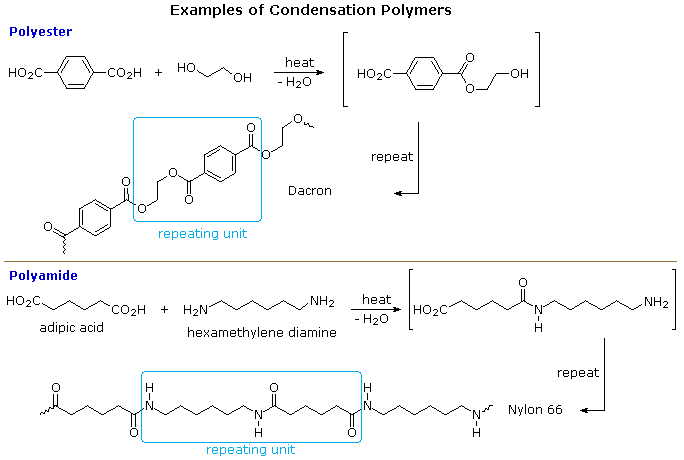
A large number of important and useful polymeric materials are not formed by chain-growth processes involving reactive species such as radicals, but proceed instead by conventional functional group transformations of polyfunctional reactants. These polymerizations often (but not always) occur with loss of a small byproduct, such as water, and generally (but not always) combine two different components in an alternating structure. The polyester Dacron and the polyamide Nylon 66, shown here, are two examples of synthetic condensation polymers, also known as step-growth polymers. In contrast to chain-growth polymers, most of which grow by carbon-carbon bond formation, step-growth polymers generally grow by carbon-heteroatom bond formation (C-O & C-N in Dacron & Nylon respectively). Although polymers of this kind might be considered to be alternating copolymers, the repeating monomeric unit is usually defined as a combined moiety.
Examples of naturally occurring condensation polymers are cellulose, the polypeptide chains of proteins, and poly(β-hydroxybutyric acid), a polyester synthesized in large quantity by certain soil and water bacteria. Formulas for these will be displayed below by clicking on the diagram.
Many of the reactions involved in these polymerizations are acyl substitution reactions, which will be covered in detail in chapter 22.
Characteristics of Condensation Polymers
Condensation polymers form more slowly than addition polymers, often requiring heat, and they are generally lower in molecular weight. The terminal functional groups on a chain remain active, so that groups of shorter chains combine into longer chains in the late stages of polymerization. The presence of polar functional groups on the chains often enhances chain-chain attractions, particularly if these involve hydrogen bonding, and thereby crystallinity and tensile strength. The following examples of condensation polymers are illustrative.
Note that for commercial synthesis the carboxylic acid components may actually be employed in the form of derivatives such as simple esters. Also, the polymerization reactions for Nylon 6 and Spandex do not proceed by elimination of water or other small molecules. Nevertheless, the polymer clearly forms by a step-growth process. Some Condensation Polymers:
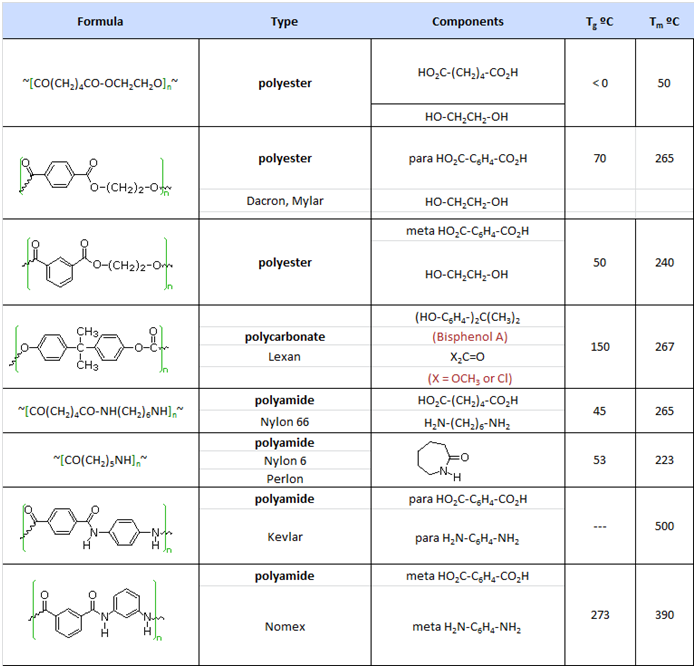
Here, Tg represents the glass transition temperature, while Tm represents the melting temperature. The difference in Tg and Tm between the first polyester (completely aliphatic) and the two nylon polyamides (5th & 6th entries) shows the effect of intra-chain hydrogen bonding on crystallinity. Kevlar and Nomex are extremely tough and resistant materials, which find use in bullet-proof vests and fire resistant clothing.
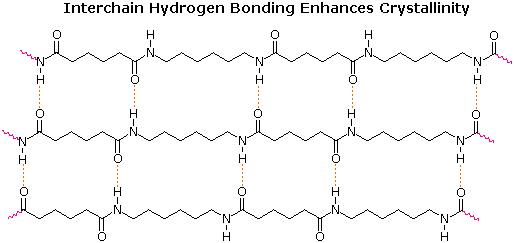
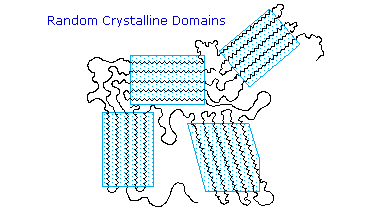
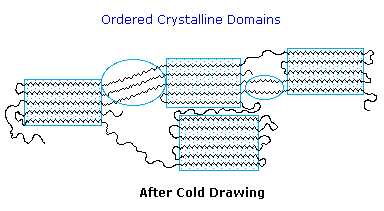
Many polymers, both addition and condensation, are used as fibers The chief methods of spinning synthetic polymers into fibers are from melts or viscous solutions. Polyesters, polyamides and polyolefins are usually spun from melts, provided the Tm is not too high. Polyacrylates suffer thermal degradation and are therefore spun from solution in a volatile solvent. Cold-drawing is an important physical treatment that improves the strength and appearance of these polymer fibers. At temperatures above Tg, a thicker than desired fiber can be forcibly stretched to many times its length; and in so doing the polymer chains become untangled, and tend to align in a parallel fashion. This cold-drawing procedure organizes randomly oriented crystalline domains, and also aligns amorphous domains so they become more crystalline. In these cases, the physically oriented morphology is stabilized and retained in the final product. This contrasts with elastomeric polymers, for which the stretched or aligned morphology is unstable relative to the amorphous random coil morphology.
This cold-drawing treatment may also be used to treat polymer films (e.g. Mylar & Saran) as well as fibers.
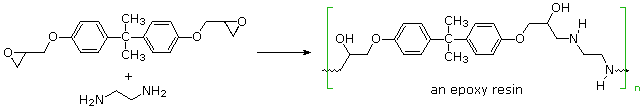
Step-growth polymerization is also used for preparing a class of adhesives and amorphous solids called epoxy resins. Here the covalent bonding occurs by an SN2 reaction between a nucleophile, usually an amine, and a terminal epoxide. In the following example, the same bisphenol A intermediate used as a monomer for Lexan serves as a difunctional scaffold to which the epoxide rings are attached. Bisphenol A is prepared by the acid-catalyzed condensation of acetone with phenol.
Video

Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Jim Clark (Chemguide.co.uk)
- Virtual Textbook of Chemistry. Authored by: William Reusch. Located at: https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/intro1.htm. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Helping you to understand Chemistry. Authored by: Jim Clark. Located at: http://www.chemguide.co.uk/. License: CC BY: Attribution