20.2: Addition of hydride reducing agents
- Page ID
- 225888
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Nucleophilic Addition of H– and R–—A Review
Addition of a hydride anion (H:–) to an aldehyde or ketone gives an alkoxide anion, which on protonation yields the corresponding alcohol. This reaction was covered previously in section 19.3. Aldehydes produce 1º-alcohols and ketones produce 2º-alcohols. In metal hydride reductions, the resulting alkoxide salts are insoluble and need to be protonated (with care) before the alcohol product can be isolated. In the sodium borohydride reduction the ethanol solvent system achieves this hydrolysis automatically. In the lithium aluminum hydride reduction water is usually added in a second step. The lithium, sodium, boron and aluminum end up as soluble inorganic salts at the end of either reaction. Note! LiAlH4 and NaBH4 are both capable of reducing aldehydes and ketones to the corresponding alcohol. 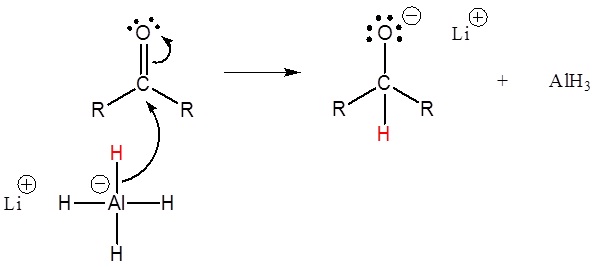
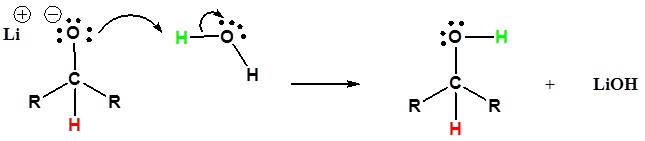
Mechanism
This mechanism is for a LiAlH4 reduction. The mechanism for a NaBH4 reduction is the same except ethanol is the proton source used in the second step.
1) Nucleophilic attack by the hydride anion

2) The alkoxide is protonated

Contributors
- Prof. Steven Farmer (Sonoma State University)
CC licensed content, Shared previously
- Nucleophilic Addition of H- and R--A Review. Authored by: Prof. Steven Farmer . Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(Smith)/Chapter_21%3A_Aldehydes_and_Ketones%E2%80%94Nucleophilic_Addition/21.8%3A_Nucleophilic_Addition_of_H%E2%80%93_and_R%E2%80%93%E2%80%94A_Review. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike

