11.1.3 Electrophilic Addition to Alkynes
- Page ID
- 22805
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Addition of strong acids to alkynes is quite similar to the addition of strong acids to alkenes. The regiochemistry follows Markovnikov’s Rule, but the stereochemistry is often different. Addition to alkenes is usually not stereospecific, whereas alkynes usually undergo anti addition.
Introduction
Strong acids usually protonate alkenes on their less substituted carbon thus developing a positive charge on the more substituted carbon of the reacting double bond. This intermediate carbocation may rearrange or simply pick up a nucleophile to form the expected addition product. The situation with alkynes is different. Similar protonation of the \(\pi\) bond of an alkyne would produce a very unstable vinyl cation so this does not happen in most cases. A vinyl cation can only have one alkyl group adjacent to the vacant p-orbital to stabilize its positive charge so the stability of the vinyl cation is similar to the mono-substituted primary alkyl cation (See below). As a result, a strong acid can form a \(\pi\) complex with the alkyne \(\pi\) bond but proton transfer to the alkyne is extremely difficult. The strong acid, however, does polarize the \(\pi\) bond developing positive charge at both carbons of the \(\pi\) bond. The more substituted carbon holds most of the positive charge and becomes the site of attack by any nucleophile present in solution. The nucleophile bonds to the more substituted carbon allowing the acid to protonate the less substituted carbon of the \(\pi\) bond.
In nucleophilic addition reactions, a nucleophile attacks an electron poor species. Now we will take a look at electrophilic addition reactions, particularly of alkynes. The reaction mechanisms, as you will notice, are quite similar to the electrophilic addition reactions of alkenes. The triple bonds of alkynes, because of its high electron density, are easily attacked by electrophiles, but less reactive than alkenes due to the compact C-C electron cloud.As with electrophilic addition to unsymmetrical alkenes, the Markovnikov rule is followed, adding the electrophile to the less substituted carbon. Here we will go through the following reactions listed below to learn the mechanisms behind these electrophilic additions of alkynes: (1) HX Addition to Alkenes, (2) Halogenation of Alkynes and (3) Hydration of Alkynes.
Figure 1
The addition of an electrophile to either an alkene or an alkyne will undergo the same steps listed below.
- Start with a reactant (either an alkene or an alkyne), which has \(\pi\) electrons. An electron pair moves from the \(\pi\) bond to the electrophilic proton to form a new covalent bond. A carbocation intermediate forms on the most stable carbon. As shown in the diagram above, the intermediate consists of hydrogen covalently bonded to the carbon and a positive charge on the other carbon.
- The addition of the halide ion to the positive charged intermediate forms the second covalent bond giving you the haloalkane product (as seen on the left of the above diagram) and a haloalkene product (as seen on the right of the above diagram). The halide ion acts as a nucleophile, which is attracted to the positive charged carbon because it has electrons to donate or share. So when the nucleophilic halide (represented as X-) attacks, it aims for the positively charged carbon resulting in that second covalent bond as seen above.
Make Note: The way to determine where the addition of the proton and the halide takes place is based on Markovnikov’s Rule, which states that the proton adds onto the carbon with the most hydrogens and the halogen prefers the most substituted carbon.
Now let's take a look at our first reaction, the addition of Hydrogen Halide.
Reaction 1: Addition of Hydrogen Halide to an Alkyne
Summary: Reactivity order of hydrogen halides: HI > HB r> HCl > HF.
Follows Markovnikov’s rule:
- Hydrogen adds to the carbon with the greatest number of hydrogens, the halogen adds to the carbon with fewest hydrogens.
- Protination occurs on the more stable carbocation. With the addition of HX, haloalkenes form.
- With the addition of excess HX, you get anti addition forming a geminal dihaloalkane.
Addition of a HX to an Internal Alkyne
As described in Figure 1, the \(\pi\) electrons will attack the hydrogen of the HBr and because this is a symmetric molecule it does not matter which carbon it adds to, but in an asymmetric molecule the hydrogen will covalently bond to the carbon with the most hydrogens. Once the hydrogen is covalently bonded to one of the carbons, you will get a carbocation intermediate (not shown, but will look the same as depicted in Figure 1) on the other carbon. Again, this is a symmetric molecule and if it were asymmetric, which carbon would have the positive charge?
The final step is the addition of the Bromine, which is a good nucleophile because it has electrons to donate or share. Bromine, therefore attacks the carbocation intermediate placing it on the highly substituted carbon. As a result, you get 2-bromobutene from your 2-butyne reactant, as shown below.
.bmp?revision=1&size=bestfit&width=442&height=160)
Now, what if you have excess HBr?
Addition due to excess HX present ? yields a geminal dihaloalkane
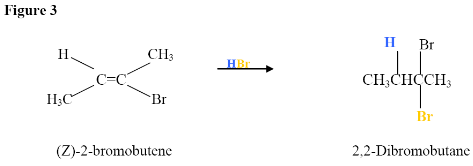
Here, the electrophilic addition proceeds with the same steps used to achieve the product in Addition of a HX to an Internal Alkyne. The \(\pi\) electrons attacked the hydrogen, adding it to the carbon on the left (shown in blue). Why was hydrogen added to the carbon on left and the one on the right bonded to the Bromine?
Now, you will have your carbocation intermediate, which is followed by the attack of the Bromine to the carbon on the right resulting in a haloalkane product.
Addition of HX to Terminal Alkyne
- Here is an addition of HBr to an asymmetric molecule.
- First, try to make sense of how the reactant went to product and then take a look at the mechanism.
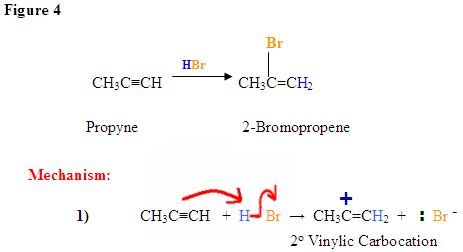
The \(\pi\) electrons are attacking the hydrogen, depicted by the electron pushing arrows and the Bromine gains a negative charge. The carbocation intermediate forms a positive charge on the left carbon after the hydrogen was added to the carbon with the most hydrogen substituents.

The Bromine, which has a negative charge, attacks the positively charged carbocation forming the final product with the nucleophile on the more substituted carbon.
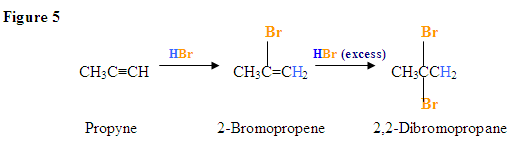
Addition due to excess HBr present
Reaction: Halogenation of Alkynes
Summary:
- Stereoslectivity: anti addition
- Reaction proceeds via cyclic halonium ion
Addition of Br2
- The addition of Br2 to an alkyne is analogous to adding Br2 to an alkene.
- Once Br2 approaches the nucleophilic alkyne, it becomes polarized.
- The \(\pi\) electrons, from the triple bond, can now attack the polarized bromine forming a C-Br bond and displacing the bromine ion.
- Now, you will get an intermediate electrophilic carbocation, which will immediately react with the bromine ion giving you the dibromo product.
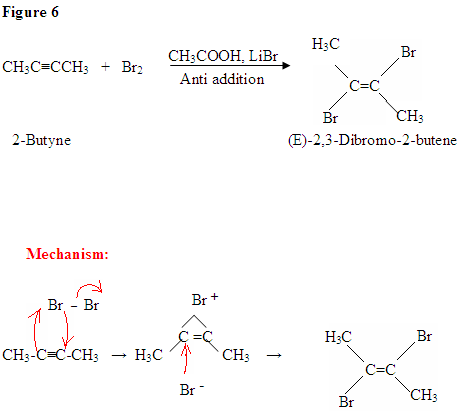
First, you see the polarized Br2 being attacked by the \(\pi\) electrons. Once you form the C-Br bond, the other bromine is released as a bromine ion. The intermediate here is a bromonium ion, which is electrophilic and reacts with the bromine ion giving you the dibromo product.
Reaction: Hydration of Alkynes
Summary: With the addition of water, alkynes can be hydrated to form enols that spontaneously tautomerize to ketones. Reaction is catalyzed by mercury ions. Follows Markovnikov’s Rule: Terminal alkynes give methyl ketones
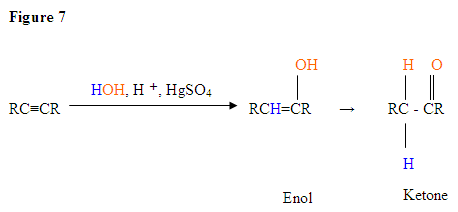
- The first step is an acid/base reaction where the \(\pi\) electrons of the triple bond acts as a Lewis base and attacks the proton therefore protinating the carbon with the most hydrogen substituents.
- The second step is the attack of the nucleophilic water molecule on the electrophilic carbocation, which creates an oxonium ion.
- Next you deprotonate by a base, generating an alcohol called an enol, which then tautomerizes into a ketone.
- Tautomerism is a simultaneous proton and double bond shift, which goes from the enol form to the keto isomer form as shown above in Figure 7.
Now let's look at some Hydration Reactions.
Hydration of Terminal Alkyne produces methyl ketones
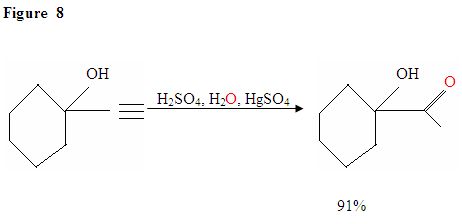
Just as described in Figure 7 the \(\pi\) electrons will attack a proton, forming a carbocation, which then gets attacked by the nucleophilic water molecules. After deprotination, we generate an enol, which then tautomerizes into the ketone form shown.
Hydration of Alkyne
As you can see here, the \(\pi\) electrons of the triple bond are attacking the proton, which forms a covalent bond on the carbon with the most hydrogen substituents. Once the hydrogen is bound you have a carbocation, which gets attacked by the water molecule. Now you have a positive charge on the oxygen which results in a base coming in and deprotinating the molecule. Once deprotonated, you have an enol, which then gets tautomerized.
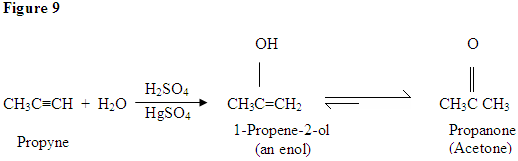
Tautomerism is shown here when the proton gets attacked by the double bond \(\pi\) electrons forming a covalent bond between the carbon and the hydrogen on the less substituted carbon. Electrons from the Oxygen end up moving to the carbon, forming a double bond with carbon and giving itself a positive charge, which then gets attacked by the base. The base deprotonates the oxygen resulting in the more stable final product at equilibrium, which is a ketone.
Outside links
References
- Vollhardt. Schore, Organic Chemistry Structure and Function Fifth Edition, New York: W.H. Freeman and Company, 2007.
- Solomons, T. W. Graham, and Craig B. Fryhle. Organic Chemistry Eighth Edition. Maryland: John Wiley and Sons Incorporated, 2008.
Practice Problems
1) Reaction: Addition of Hydrogen Halide to an Alkyne
1a) What product would result from the reaction of 1-Pentyne with Hydrogen Bromide?
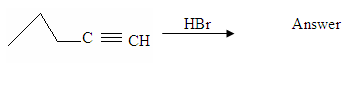
1b) Take your answer from 1a and add an excess of Hydrogen Bromide. What would be the product?
1c) What is the mechanism for this reaction?
2) Reaction: Addition of X2 to Alkynes
2a) What product would result from the reaction of 1-Pentyne with Br2?
.bmp?revision=1&size=bestfit&width=382&height=74)
2b) Take your answer from 2a and add an excess of Br2. What would be the product?
2c) What is the mechanism for this reaction?
3) Reaction: Hydration of Alkynes
3a) If 1-Pentyne were to react with mercury(I) sulfate, water, and sulfuric acid, what would be the product?
4) What is the product when 3-methylbutyne reacts with HCl? Include in your answer:
a) the reaction mechanism
b) a clear explanation for why the hydrogen and chlorine bind to where they do
5) a) Draw the enol form of the Ketone below.
b) Draw the Keto form from the enol below.
Contributors
- Prof. Hilton Weiss (Bard College)
- Aleksandra Milman
Further Reading
MasterOrganicChemistry
Alkyne Reactions Patterns - The Carbocation Pathway
Alkyne Reactions Patterns - The 3-Membered Ring Pathway
Carey 5th Ed Online
Khan Academy

