12.1: Radical Intermediates
- Page ID
- 365789
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In organic chemistry, we say that there are generally four types of intermediates: carbocations, carbanions, carbenes, and radicals. We have not said much about radicals up to this point, except that they are carbon centers that have an unpaired electron.
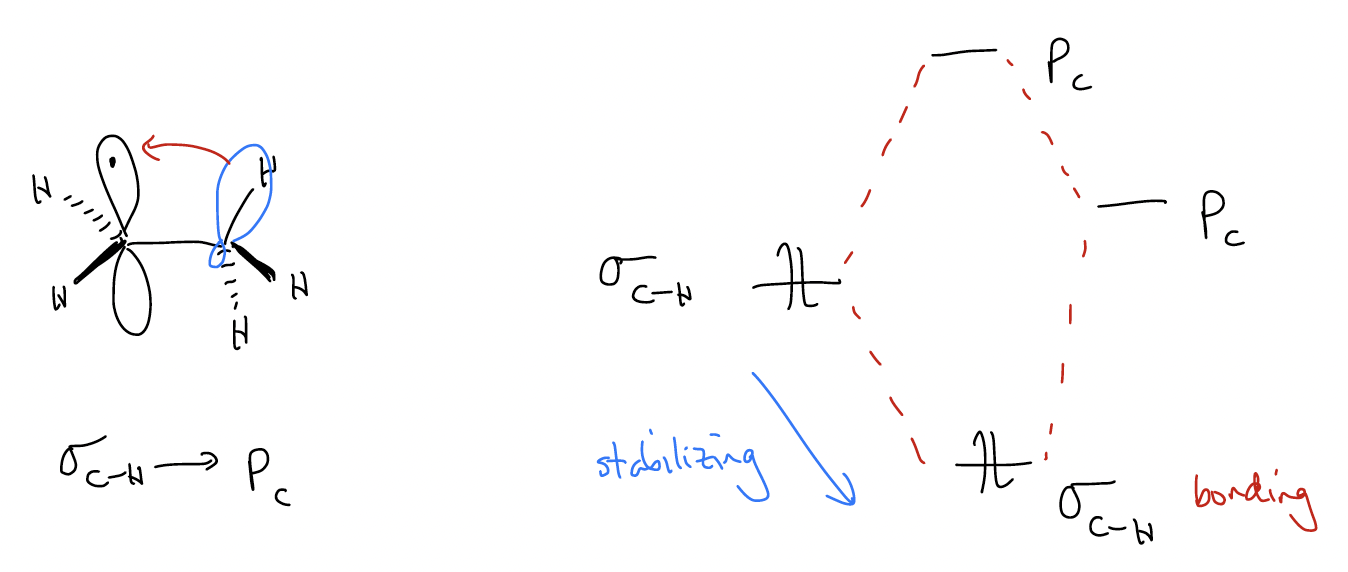
A free radical is an intermediate that is electron-deficient, but unlike the carbocation, it is overall neutral. We generally say that the carbon is sp2-hybridized and has a trigonal planar geometry (although in some cases it is more like a very shallow trigonal pyramid). The unpaired electron sits in an atomic p orbital perpendicular to the trigonal plane. Like carbocations, the radical can be stabilized by additional alkyl groups (s-donation) such that 3° radicals are more stable than 2° radicals, etc. Similarly, benzylic and allylic radicals can be stabilized by resonance delocalization and are more stable than those radicals with \(σ\)-donation alone.

Why would radicals be stabilized by these effects? Let’s look at the molecular orbital diagram for a radical intermediate that undergoes \(σ\)-donation. In this case, we have a three-electron system in which the unpaired electron in the atomic p orbital is overlapping with a pair of \(σ\) bonding electrons. This overlap creates two new molecular orbitals. The new bonding orbital (with two electrons) is lower in energy, and the new antibonding orbital (with one electron) is higher in energy. This is certainly destabilizing to the unpaired electron, but the overall system is still stabilized because two electrons are able to occupy the low-energy bonding orbital.
Before we talk about their reactivity, we first need to understand how radicals are formed in the first place. Recall that, according to molecular orbital theory, a \(σ\) bond is formed from the overlap of two electrons, where one electron is contributed from each atom. It might seem possible, then, that we could break a \(σ\) bond homolytically to generate two radicals. In a homolytic cleavage, a \(σ\) bond is broken equally so that each atom receives one electron, thus forming two new radicals. We use singly-headed curly arrows to describe this type of cleavage. The energy required to break a bond homolytically is called the bond dissociation energy (BDE), and is usually lower than heterolytic cleavage because no charges are generated.

The simplest method of inducing homolytic cleavage is called pyrolysis (or thermolysis), which increases the bond vibration amplitude so much that the s bond is broken homolytically. For ethane, the \(σ\)C-C BDE is 90 kcal/mol, the result of which is two methyl radicals. Since pyrolysis involves high temperatures, some functional groups will not survive, and thus we must resort to other methods of generating radicals. These methods involve modifying the system so that the BDE is much lower.
So, how can we modify an alkane in order to make the \(σ\)C-C bond weaker (lower BDE)? One easy way is to introduce some strain into the alkane so that the \(σ\)C-C bond is easily broken, releasing any strain energy. This happens readily for cyclopropyl rings. Pyrolysis forms a 1,3-diradical, this time with a BDE of only 65 kcal/mol. The increased strain of the cyclopropane ring made it less stable, and thus more likely to cleave homolytically.

But this is somewhat limited in scope because not all synthetically useful starting materials have cyclopropane rings. What if, instead of making the starting material less stable, we made the resulting radicals more stable? If the resulting radicals that are formed via homolytic cleavage are more stable, then the initial \(σ\)C-C bond should be weaker. This is the case for allylic systems. For example, the BDE of 1-butene is 76 kcal/mol because homolytic cleavage of the \(σ\)C-C bond generates a methyl radical and a resonance-stabilized allyl radical. The transition state energy is much lower, resulting in a lower BDE.

Another technique for generating radicals is to start with molecules containing especially weak bonds to begin with. You may recall that the \(σ\)O-O bond is very weak. Simple peroxides have \(σ\)O-O bonds with BDEs of ~38 kcal/mol, and cleave to produce alkoxy radicals. When an acyl (C=O) group is added to the peroxide, as in acyl peroxides, the BDE is lowered even more to ~29 kcal/mol (think mCPBA). The result is an alkoxy radical and a carboxyl radical, the latter of which further decomposes to produce CO2 and an alkyl radical. Azo compounds, which contain an internal N=N bond, readily produce radicals because a molecule of N2 gas is generated. While this can be very hazardous, a more common source of radicals generated by N2 extrusion is azobisisobutyronitrile (AIBN), which has a BDE of 31.9 kcal/mol.

Once they are generated, there are several reaction pathways available for radicals:
1. Abstraction: the process by which a radical species takes a hydrogen atom (NOT a proton) from another molecule.

2. Disproportionation: the process by which a radical abstracts a hydrogen from the vicinal (adjacent) position of another radical to produce an alkane and an alkene.

3. \(β\)-cleavage: the process by which a radical decomposes to give an alkene and a new radical.

4. Dimerization: the process by which two radicals react with each other to create an alkane.
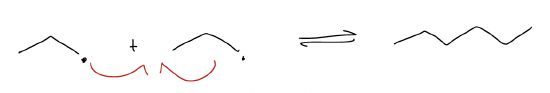
5. Addition: the process by which a radical adds to a \(π\) bond or cleaves a \(σ\) bond to generate a new radical.

We will see nearly all of these types of radical processes in the reactions that follow in the remainder of this chapter.

