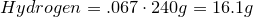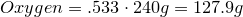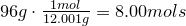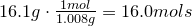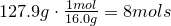Template:HideTOC
These are homework exercises to accompany the Textmap created for Chemistry: A Molecular Approach by Nivaldo Tro. Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis; please contact Delmar Larsen for an account with access permission.
Q5.29
Classify each compound as ionic or molecular
- \(Fe_2O_3\)
- \(SO_4\)
- \(H_2O\)
- \(NaCl\)
Strategy
- First you need to have good understanding of the difference between Ionic and Molecular.
- Ionic compound is formed between a metal and a non-metal
- Molecular compound is formed between two non-metals
- Then you need to figure out which elements are metals and non-metals by looking at the periodic table
Answers
- Ionic
- Molecular
- Molecular
- Ionic
Q5.33
Question: How many types of each atom are in each formula?
- \((NH_4)_2SO_4\)
- \(CaCO_3\)
- \(Mg(OH)_2\)
- \(NaHCO_3\)
What We Know: A chemical formula gives the number of atoms in a compound and the element ratio within a compound.
What It's Asking For: Determine the number of each atom in the given compounds.
Strategy: The subscripts after each atom give the number of atoms in the compound. If the subscript is outside of the parenthesis, the subscript is applied to each atom within the parenthesis.
Solution:
- 2 N, 8 H, 1 S, 4 O
- 1 Ca, 1 C, 3 O
- 1 Mg, 2 O, 2 H
- 1 Na, 1 H, 1 C, 3 O
Q5.48
Write four different ionic compounds that can be formed using the following elements: Sodium, Chlorine, Aluminum and Sulfur.
Strategy:
-
Identify which elements are the metals and which are the nonmetals that will be arranged to form the ionic compounds.
-
Ionic compounds always contain positive and negative ions; therefore identify the positive charge of each metal identified in the above step and the negative charge of each nonmetal identified in the above step using the Periodic Table for reference.
-
Combine each metal with each nonmetal to form the initial foundation for the ionic compound. The sum of the positive charges must equal the sum of the negative charges within the compound which means that the number of each ion may have to be adjusted to achieve the balance of ions. If so, then the formula of the ionic compound will always reflect the smallest whole number ratio of ions. For example: 2:1, 2:3, etc.
Solution
- \(NaCl\)
- \(Na_2S\)
- \(AlCl_3\)
- \(Al_2S_3\)
Q5.49
Write the formula for the compound that forms between Potassium and each of the polyatomic ions listed below:
- Sulfate
- Perchlorate
- Nitrate
- Phosphate
Strategy
- Identify the compound listed
- Write out the chemical equation for each reaction
- Balance the equations
Hints
- The formula for Sulfate is SO42-
- The formula for Perchlorate is ClO4-
- The formula for Nitrate is NO3-
- The formula for Phosphate is PO43-
Solution
- \(2K^+ + SO_4^{2-} \rightarrow K_2SO_4\)
- \(K^+ + ClO_4^- \rightarrow K_2CLO_4\)
- \(K^+ + NO_3^- \rightarrow KNO_3\)
- \(3K^+ + PO_4^{3-} \rightarrow K_3PO_4\)
Q5.60
Write the name from the formula of the formula from the name for each hydrated ionic compound
- Iron(III) phosphate tetrahydrate
- \(CsBr \cdot 4H_2O\)
- Cobalt(II) chloride hexahydrate
- \(LiI \cdot 3H_2O\)
Strategy
- Name the cation and anion combination in the first part of the equation, or if the name is given, name the element combination with the anion first, then the cation. The anion is capitalized, while the cation remains lowercase.
- If the compound involves a metal that forms more than one cation, the number of cations it forms goes in between parentheses in roman numeral form. If the compound does not involve a metal, than list cation charges as normal.
- For each hydrate at the end of the compound, the prefix at the beginning indicates the number of hydrates. Name the appropriate prefix or number needed for each formula or compound name. Make sure the hydrate is lowercase.
Solutions
(1) Iron(III) phosphate tetrahydrate
- Iron phosphate as an element is written as FePO4, since both parts have a charge of 3, they are not needed in the equation.
- The prefix tetra stands for "4", so therefore tetrahydrate as an element is written as 4H2O
- The two parts combined make the final formula \(FePO_4 \cdot 4H_2O\)
(2) \(CsBr \cdot 4H_2O\)
- Cs and Br with 1 charge stands for Cesium Bromide
- 4 as a prefix is Tetra, so the end of the formula is tetrahydrate
- The final name of the compound is Cesium bromide tetrahydrate
(3) Cobalt(II) chloride hexahydrate
- Cobalt Chloride has a compound formula of \(CoCl\), and with Cobalt's charge of 2 involved, a subscript of 2 is added to the end to make \(CoCl_2\)
- The "hexa" prefix stands for 6, meaning there are 6 hydrates or 6H2O
- The two parts put together makes the final compound, \(CoCl_2 \cdot 6H_2O\)
(4) \(LiI \cdot 3H_2O\)
- Li and I both with single charges make up the compound Lithium iodide
- 3 as a prefix i labled as "tri", so with 3 hydrates, the compound is named as trihydrate
- The final compound put together makes Lithium iodide trihydrate
Help with naming compounds: http://chemwiki.ucdavis.edu/Wikitext...onic_Compounds
Q5.65
Write the name for each molecular compound.
- \(CO\)
- \(H_2S\)
- \(SF_6\)
- \(N_2O_2\)
Strategy
First, you have to know how to name a molecular compound. To name a compound, its the prefix, name of first element, prefix and name of the second element with the suffix -ide. The prefixes are as follows:
- mono=1
- di=2
- tri=3
- tetra=4
- penta=5
- hexa=6
- hepta=7
- octa=8
- nona=9
- deca=10
After finding the elements and suffixes, just put it together and you have a molecular compound.
Solution
- CO: Since we only have one of each, the carbon does not have a suffix, so we would just use Carbon and there is only one oxygen so the suffix is mono. So the answer is Carbon Monoxide
- H2S: The hydrogen has a coefficient of 2, so the suffix would be di and the sulfur has no coefficients. Therefore, the answer would be dihydrogen monosulfide.
- SF6: The sulfur has no coefficients, so it does not change. The fluoride has a coefficient of 6. Therefore, the answer is Sulfur Hexafluoride
- N2O2: The nitrogen has a coefficient of 2, while the oxygen has a coefficient of 3. The compounds name is Dinitrogen trioxide.
Q5.75a
Find the amount of moles contained in each specimen.
- 7.87 kg H2O2
- 2.34 kg NaCl
- 12.5 g C2H6O
- 85.72 g NH3
Strategy
A. First, determine the units that are given for each sample. In order to convert these measurements to moles, each sample should first be written in grams. Two of the given samples are measured in kilograms. Use the following conversion factor to convert kilograms to grams, with \(x\) representing the given mass:
\[x\, kg\cdot \dfrac{1000\, g}{1\, kg}\]
B. Next, find the atomic masses of all the atoms in each compound by using the Periodic Table. Then, add these atomic masses together for each compound. The resulting value will be the number of grams of each sample that make up one mole.
C. Convert the mass in grams of each sample to moles by multiplying it by the following conversion factor, called the molar mass, with x representing the mass of the given specimen and \(y\) representing the calculated atomic mass found in step B:
\[x\, g\cdot \dfrac{1\, mole}{y\, g}\]
A Numbers 1 and 2 first need to be converted into grams.
- \[7.87\, kg\, H_2O_2\cdot \dfrac{1000\, g}{1\, kg}=7,870\, g\, H_2O_2\]
- \[2.34\, kg\, NaCl\cdot \dfrac{1000\, g}{1\, kg}=2,340\, g\, NaCl\]
B The following are the calculated molar masses of each of the given compounds. The atomic mass of each element has been rounded to 4 significant figures in these calculations.
- H2O2: \[2\, (1.008\, g\, H)+2\, (16.00\, g\, O)=\dfrac{34.02\, g}{1\, mole}\, H_2O_2\]
- NaCl: \[(22.99\, g\, Na)+(35.45\, g\, Cl)=\dfrac{58.44\, g}{1\, mole}\, NaCl\]
- C2H6O: \[2\, (12.01\, g\, C)+6\, (1.008\, g\, H)+\left ( 16.00\, g\, O \right )=\dfrac{46.07\, g}{1\, mole}\, C_2H_6O\]
- NH3: \[(14.01\, g\, N)+3\, (1.008\, g\, H)=\dfrac{17.03\, g}{1\, mole}\, NH_3\]
C The number of moles in each specimen can now be calculated by multiplying the mass in grams of each sample by its molarity.
- \[7,870\, g\, H_2O_2\cdot \dfrac{1\, mole\, H_2O_2}{34.02\, g\, H_2O_2}=231\, moles\, H_2O_2\]
- \[2,340\, g\, NaCl\cdot \dfrac{1\, mole\, NaCl}{58.44\, g\, NaCl}=40.0\, moles\, NaCl\]
- \[12.5\, g\, C_2H_6O\cdot \dfrac{1\, mole\, C_2H_6O}{46.07\, g\, C_2H_6O}=0.271\, moles\, C_2H_6O\]
- \[85.72\, g\, NH_3\cdot \dfrac{1\, mole\, NH_3}{17.03\, g\, NH_3}=5.033\, moles\, NH_3\]
Q5.75b
Calculate the number of moles in each of the following examples.
- 402.5 mg of NO2
- 2.7 kg of H2O
- 323 g of CBr4
- 2.9 kg of CaO
Solution
a)
First, calculate the molar mass of NO2.
14.007g/mol N + 2 (15.999 g/mol O) = 46.0055 g/mol
Now, convert the sample from mg to g.
402.5 mg NO2 |
1 g |
0.4025 g NO2 |
| |
1000 mg |
|
Finally, use the molar mass to determine how many moles your sample size contains.
| 0.4025 g NO2 |
1 mol |
0.0087 moles of NO2 |
| |
46.0055 g |
|
This gives us the final answer, 0.0087 moles of NO2.
From this, we can deduce that:
\[\text{Moles of substance} = \text{Mass of substance (g)}{Molar mass of substance}\]
b) 150 moles of H2O
c) 0.974 moles of CBr4
d) 52 moles of CaO
Q5.80
For each substance, convert the given molecules to mass in grams:
- 3.2 x 1024 Cl2 molecules
- 8.25 x 1018 CH2O molecules
- 1 carbon dioxide molecule
Strategy:
Step 1: Convert the given molecules to moles by dividing by Avogadro's number.
Step 2: Multiply the number of moles by the Molar Mass of the substance to determine the grams.
Step 3: Through correct Dimensional Analysis, the molecules and moles will cancel to leave grams.
\[\dfrac{molecules}{Avogadro's}\rightarrow moles\rightarrow moles \cdot MM\rightarrow grams\]
Helpful Hints:
- Avogadro's number: 6.022 x 1023 mol of a substance
- Molar mass (MM): the weight of a substance in the units g/mol, found by adding the atomic masses of each element.
- Example: MM of H2O --> H(1.008 x 2) + O(15.999)= 18 g/mol
Solution
a) 3.8 x 1051 grams
b) 4.11 x 1045 grams
c) 7.31 x 1025 grams
Q5.105
Write the empirical formula and molecular formula for the compound with the given percent composition and molecular weight.
- 40.0% Carbon
- 6.70% Hydrogen
- 53.3% Oxygen
Molecular Weight ≈ 240 g/mol
Solution
Strategy:
- Calculate amount of grams of each element we have.
- Convert amount of grams into number of moles of each element.
- Obtain molecular formula.
- Find the common factor among the number of atoms each elements has, then obtain the empirical formula.
Solution:
1. Calculate amount of grams in each element:

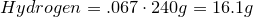
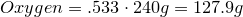
2. Convert amount of grams into number of moles of each elements:
Carbon-
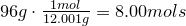
Hydrogen-
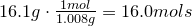
Oxygen-
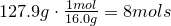
3. Obtain molecular formula:
\[C_8H_{16}O_8\]
4. Find common factor and obtain empirical formula:
Common factor is 8
Empirical formula is
\[CH_2O\]
Q5.106
For several compounds both the molar mass and and empirical formulas are listed. What is the molecular formula for each compound.
- C2OH4, 88 g/mol
- C2H8N, 46 g/mol
- NH2, 32 g/mol
Strategy
- A. Find the empirical formula mass of the compound
- B. Determine the ratio between the empirical formula mass and the molar mass of the compound
- C.Multiply the empirical formula by the factor found in part B.
Solution
C2OH4, 88 g/mol
A.
Carbon: 2(12.0g/mol)
Oxygen: 1(16.0g/mol)
Hydrogen: 4(1.0g/mol)
=44.0 g/mol
B.
\[\dfrac{88.0g/mol}{44.0g/mol}=2\]
C.
2(C2OH4)= C4O2H8
C2H8N, 46 g/mol
A.
Carbon: 2(12.0g/mol)
Hydrogen: 8(1.0g/mol)
Nitrogen: 1(14.0g/mol)
=46.0 g/mol
B.
\[\dfrac{46.0g/mol}{46.0g/mol}=1.0\]
C.
1(C2H8N)=C2H8N
NH2
A.
Nitrogen: 1(14.0g/mol)
Hydrogen: 2(1.0g/mol)
=16.0g/mol
B.
\[\dfrac{32.0g/mol}{16.0g/mol}=2\]
C.
2(NH2)=N2H4
Q5.107
After the combustion of a hydrocarbon, we find 66.02g of CO2 and 27.02g of H2O. What is the empirical formula of this hydrocarbon?
Solutions
66.02 x 1 mol/ 44.0 g= 1.500 mol CO2= 1.5 mol C
27.02 x 1 mol/ 18g = 1.501 mol H20= 3 mol H
C1.5 H3= C3 H6
You start by taking both, divide by the molar mass, use the mol ratio of the compound and multiple to get rid of decimals.
Additional Question
Additional Questions 1. For the following molecules; write the chemical formula, determine how many atoms are present in one molecule, determine the molecular weight, determine the number of moles in exactly 1 gram, and the number of moles in exactly 10-6 gram. a. carbon dioxide b. iron (II) chloride c. dinitrogen pentoxide d. iron (III) sulfate 2. Name the following compounds, determine the molecular weight, determine the percent composition, and determine how many moles in 8.35 grams of the compound. a. KI b. CaF2 c. Cu2SO4 d. N2O e. LiOH 3. Give the chemical formula (or atomic symbol), molecular (or atomic) weight, and charge for the following ions: a. sulfate b. sulfite c. nitrate d. chloride e. nitride f. acetate g. carbonate 4. Calculate the number of moles in: a. 20.1797 g of Ne b. 45.3594 g of Ne c. 0.198669 g of Ne 5. Calculate the mass of: a. 2.00 mole of Fe b. 4.362 x 10-5 mol of Fe c. 4.362 x 10-5 mol of Li