8.3: Chemical Formulas as Conversion Factors
- Page ID
- 367059
- Use chemical formulas as conversion factors.
- Given a compound formula and the mass of the substance calculate the mass of constituent elements and number of component particles (elements or ions).
Figure \(\PageIndex{1}\) shows that we need 2 hydrogen atoms and 1 oxygen atom to make one water molecule. If we want to make two water molecules, we will need 4 hydrogen atoms and 2 oxygen atoms. If we want to make five molecules of water, we need 10 hydrogen atoms and 5 oxygen atoms. The ratio of atoms we will need to make any number of water molecules is the same: 2 hydrogen atoms to 1 oxygen atom.
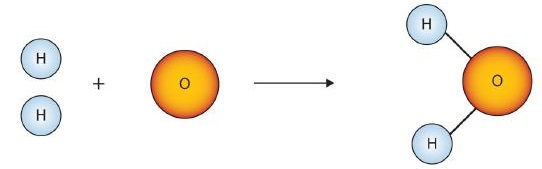
Using formulas to indicate how many atoms of each element we have in a substance, we can relate the number of moles of molecules to the number of moles of atoms. For example, in 1 mol of water (H2O) we can construct the relationships given in (Table \(\PageIndex{1}\)).
| 1 Molecule of \(H_2O\) Has | 1 Mol of \(H_2O\) Has | Molecular Relationships |
|---|---|---|
| 2 H atoms | 2 mol of H atoms | \(\mathrm{\dfrac{2\: mol\: H\: atoms}{1\: mol\: H_2O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: H_2O\: molecules}{2\: mol\: H\: atoms}}\) |
| 1 O atom | 1 mol of O atoms | \(\mathrm{\dfrac{1\: mol\: O\: atoms}{1\: mol\: H_2O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: H_2O\: molecules}{1\: mol\: O\: atoms}}\) |
A mole represents a very large number! The number 602,214,129,000,000,000,000,000 looks about twice as long as a trillion, which means it’s about a trillion trillion.
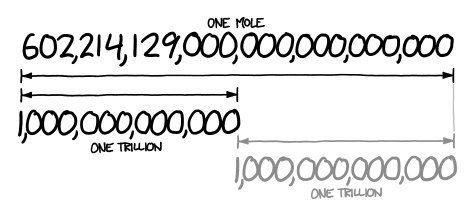
(CC BY-SA NC; https://what-if.xkcd.com/4/).
A trillion trillion kilograms is how much a planet weighs. If 1 mol of quarters were stacked in a column, it could stretch back and forth between Earth and the sun 6.8 billion times.
| 1 Molecule of \(C_2H_6O\) Has | 1 Mol of \(C_2H_6O\) Has | Molecular and Mass Relationships |
|---|---|---|
| 2 C atoms | 2 mol of C atoms | \(\mathrm{\dfrac{2\: mol\: C\: atoms}{1\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: C_2H_6O\: molecules}{2\: mol\: C\: atoms}}\) |
| 6 H atoms | 6 mol of H atoms | \(\mathrm{\dfrac{6\: mol\: H\: atoms}{1\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: C_2H_6O\: molecules}{6\: mol\: H\: atoms}}\) |
| 1 O atom | 1 mol of O atoms | \(\mathrm{\dfrac{1\: mol\: O\: atoms}{1\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: C_2H_6O\: molecules}{1\: mol\: O\: atoms}}\) |
|
2 (12.01 amu) C 24.02 amu C |
2 (12.01 g) C 24.02 g C |
\(\mathrm{\dfrac{24.02\: g\: C\: }{1\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: C_2H_6O\: molecules}{24.02\: g\: C\: }}\) |
|
6 (1.008 amu) H 6.048 amu H |
6 (1.008 g) H 6.048 g H |
\(\mathrm{\dfrac{6.048\: g\: H\: }{1\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: C_2H_6O\: molecules}{6.048\: g\: H\: }}\) |
|
1 (16.00 amu) O 16.00 amu O |
1 (16.00 g) O 16.00 g O |
\(\mathrm{\dfrac{16.00\: g\: O\: }{1\: mol\: C_2H_6O\: molecules}}\) or \(\mathrm{\dfrac{1\: mol\: C_2H_6O\: molecules}{16.00\: g\: O\: }}\) |
The following example illustrates how we can use the relationships in Table \(\PageIndex{2}\) as conversion factors.
If a sample consists of 2.5 mol of ethanol (C2H6O), how many moles of carbon atoms does it have?
Solution
| Steps for Problem Solving |
If a sample consists of 2.5 mol of ethanol (C2H6O), how many moles of carbon atoms does it have? |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." |
Given: 2.5 mol C2H6O |
| List other known quantities. |
1 mol C2H6O = 2 mol C |
|
Prepare a concept map and use the proper conversion factor. |
|
| Cancel units and calculate. |
Note how the unit mol C2H6O molecules cancels algebraically. \(\mathrm{2.5\: \cancel{mol\: C_2H_6O\: molecules}\times\dfrac{2\: mol\: C\: atoms}{1\: \cancel{mol\: C_2H_6O\: molecules}}=5.0\: mol\: C\: atoms}\) |
| Think about your result. | There are twice as many C atoms in one C2H6O molecule, so the final amount should be double. |
If a sample contains 6.75 mol of Na2SO4, how many moles of sodium atoms, sulfur atoms, and oxygen atoms does it have?
- Answer
- 13.5 mol Na atoms, 6.75 mol S atoms, and 27.0 mol O atoms
Once mass or moles of a compound is converted to the moles of a component an element or ion , Avogadro's number can be used to find the number of individual atoms or ions. This calculalculation, like the other ones covered in this section, can also be done in reverse.
What mass of aluminum oxide do you need to measure out to have a sample with \(4.32 \times 10^{21}\) aluminum ions?
Solution
| Steps for Problem Solving |
What mass of aluminum oxide do you need to measure out to have a sample with \(4.32 \times 10^{21}\) aluminum ions? |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." |
Given: \(4.32 \times 10^{21}\) Al3+ |
| List other known quantities. |
1 mol Al3+ = \(6.022 \times 10^{23}\) Al3+ 1 mol Al2O3 = 2 mol Al3+ 1 mol Al2O3 = 101.96 g Al2O3 |
|
Prepare a concept map and use the proper conversion factor. |
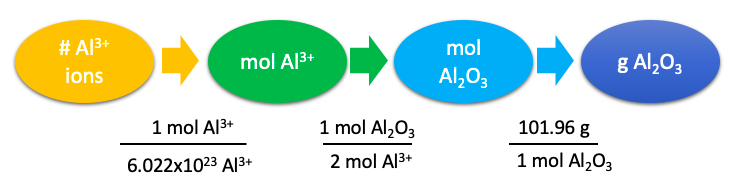 |
| Cancel units and calculate. |
\(\require{cancel}\mathrm{4.32\times 10^{21}\: \cancel{\:Al^3+}\times\dfrac{1\: \cancel{mol\: Al^3+}}{6.022\times 10^{23}\:\cancel{\: Al^3+}}\times\dfrac{1\: \cancel{mol\:Al_2O_3}}{2\: \cancel{mol\: Al^3+}}\times\dfrac{101.96\: g\: Al_2O_3}{1\: \cancel{mol\:Al_2O_3}}=0.366\: g\: Al_2O_3}\) |
What number of carbon atoms is present in 82.17 g of C4H10?
- Answer:
- \(3.406 \times 10^{23}\) C
Alternatively, once you convert from mass of a compound to moles of a particular element, you can use the moles of the element to find the mass contributed to the total by that element alone. This calculation can be done in reverse as well.
Determine the mass of Oxygen in 75.0g of C2H6O.
Solution
| Steps for Problem Solving |
Determine the mass of Oxygen in 75.0g of C2H6O |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." |
Given: 75.0g C2H6O |
| List other known quantities. |
1 mol O = 16.0g O 1 mol C2H6O = 1 mol O 1 mol C2H6O = 46.07g C2H6O |
|
Prepare a concept map and use the proper conversion factor. |
|
| Cancel units and calculate. |
\(\require{cancel}\mathrm{75.0\: \cancel{g\: C_2H_6O}\times\dfrac{1\: \cancel{mol\: C_2H_6O}}{46.07\:\cancel{g\: C_2H_6O}}\times\dfrac{1\: \cancel{mol\:O}}{1\: \cancel{mol\:C_2H_6O}}\times\dfrac{16.00\: g\: O}{1\: \cancel{mol\:O}}=26.0\: g\: O}\) |
| Think about your result. | The mass of oxygen has to be less than the total mass of the ethanol. |
What mass of sodium sulfate contains 18.0 g of sodium?
- Answer:
- 55.6 g Na2SO4

