7.3: The Dissolving Process- Ionic Compounds Versus Covalent Compounds
- Page ID
- 366536
- Differentiate between aqueous solutions of ionic and covalent compounds.
The Process of Dissolution
Water dissolves many ionic compounds and some covalent compounds. The mechanism by which ionic compounds and covalent compounds dissolve, however, is different.
Water molecules move about continuously due to their kinetic energy. When a crystal of sodium chloride is placed into water, the water's molecules collide with the crystal lattice. Recall that the crystal lattice is composed of alternating positive and negative ions. Water is attracted to the sodium chloride crystal because water has both a slightly positive end (the hydrogens) and a sightly negative end (the oxygen). The positively charged sodium ions in the crystal attract the oxygen end of the water molecules. The negatively charged chloride ions in the crystal attract the hydrogen end of the water molecules. The action of the water molecules takes the crystal lattice apart (see image below).
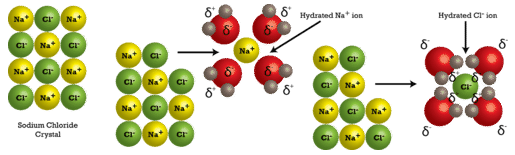
After coming apart from the crystal, the individual ions are then surrounded by water molecules. Note that the individual \(\ce{Na^+}\) ions are surrounded by water molecules with the oxygen atom oriented near the positive ion. Likewise, the chloride ions are surrounded by water molecules with the opposite orientation. Ions being surrounded by water molecules helps to stabilize aqueous solutions by preventing the positive and negative ions from coming back together and reforming a crystal.
Table sugar is sucrose \(\left( \ce{C_{12}H_{22}O_{11}} \right)\), and is an example of a covalent compound. Solid sugar consists of individual sugar molecules held together by relatively weak forces. When water dissolves sugar, it separates the individual sugar molecules by disrupting the relatively weak forces between molecules, but it does not break the covalent bonds between the carbon, hydrogen, and oxygen atoms within the molecule. The attractive forces between water molecules and solute molecules are also different than the attractive forces between water molecules and hydrated ions. This topic will be discussed in more depth in CH222.

