6.1: Ions
- Page ID
- 366501
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Predict the charge of monatomic main group ions.
- Given the symbol for a monatomic, fixed-charge ion write the systematic name.
- Given the systematic name for a monatomic, fixed-charge ion write the symbol.
- Move between a symbol or name for a monatomic, fixed-charge ion and the number of electrons.
Cations and Anions
Recall that neutral atoms have the same number of protons and electrons. In nature, however, many atoms lose or gain electrons and change from being neutral to having a net positive or negative charge. An atom that picks up a charge by gaining or losing electrons is called an ion. In this section we will focus on monatomic ions: ions formed from a single atom. Later in this chapter we will look at a second type of ion called a polyatomic ion; these form when a group of bonded atoms collectively gain a charge.
Metals have a strong tendency to lose electrons. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The atom therefore now has a positive charge because of the overabundance of protons. Any ion with a positive charge is referred to as a cation. Nonmetals, on the other hand, tend to gain electrons. This means the ion formed from a nonmetal atom with end up with more electrons than protons and have an overall negative charge. An anion is the general name given to any negatively charged ion. Hydrogen behaves a little differently than the other nonemtals. Most often it loses its only electron and becomes a cation. There are some rare cases, however, where it can gain an electron an become an anion.
The names for positive and negative ions are pronounced CAT-eye-ons (cations) and ANN-eye-ons (anions), respectively.
Formation of Ions
For any ion, we can find the charge by taking the number of electrons and subtracting it from the number of protons. We will first look at a sodium atom and a sodium ion to understand this relationship.
Sodium atoms have 11 protons (because the atomic number of sodium is 11) and 11 electrons (because the number of electrons must equal the number of protons in a neutral atom). Subtracting 11 (the number of electrons) from 11 (the number of protons) gives zero, the overall charge on the neutral atom. See the right side of Figure \(\PageIndex{1}\) .
Sodium atoms always lose one electron when they become ions. This means they still have 11 protons, but now have only 10 electrons. Since 11 minus 10 is one, the overall charge on the ion is +1. You always need to indicate the sign (+ or -) when writing charges. Additionally, to differentiate a sodium atom from a sodium ion, both the chemical formula and the name change. The name change is rather trivial for cations: you just add the word ion after the name of the element. For the chemical formula of an ion, the charge is indicated above and to the right of the symbol for the element. The chemical formula for a sodium ion is therefore Na+. When the charge is plus one, the one is left out of the chemical formula. To give another example, calcium atoms always lose two electrons when they form ions. The name of the resulting ion is a calcium ion and its chemical formula is Ca2+.

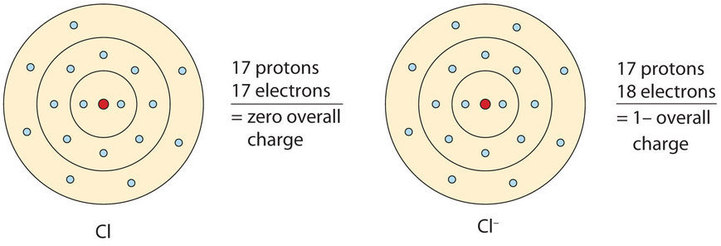
Now, let's consider a chlorine atom. A chlorine atom always gains one electron when it forms an ion (Figure \(\PageIndex{2}\)). A chlorine atom starts with 17 electrons and 17 protons and is neutral. After gaining an electron to become an ion, it now has 18 electrons. The charge is determined by taking 17 (the number of protons) and subtracting 18 (the number of electrons); it is equal to -1. We have to include the sign to indicate that it is negative.
The chemical formula for a chlorine atom is Cl and the chemical formula for the ion is Cl−. Notice again how the one is left off of the ion charge when writing the formula. Anion names work slightly differently than cation names: the ion formed from a chlorine atom is called a chloride ion. For monatomic anions, the suffix -ide is added onto the root name of the element to create the name of the anion. Oxygen atoms, for example, become oxide ions (O2-) and phosphorus atoms become phosphide ions (P3-).
For each ion, determine its name and the number of electrons it contains.
- Mg2+
- N3−
- F-
Solution
(a) Since Mg2+ is a cation, its name is the name of the element it comes from plus the word ion. This would make it a magnesium ion. Magnesium atoms contain 12 protons in their nucleus. To get a +2 charge, the ion had to lose two electrons. A magnesium ion therefore has 10 electrons.
(b) Since N3− is an anion, its name is the root name of the element with the suffix -ide. This would make it a nitride ion. Nitrogen atoms contain 7 protons in their nucleus. To get a -3 charge, the ion had to gain three electrons. A nitride ion therefore has 10 electrons.
(b) Since F− is an anion, its name is the root name of the element with the suffix -ide. This would make it a fluoride ion. Fluorine atoms contain 9 protons in their nucleus. To get a -1 charge, the ion had to gain one electron. A fluoride ion therefore has 10 electrons.
Exercise \(\PageIndex{1}\)
For each ion, determine its name and the number of electrons it contains.
- S2−
- Al3+
- Rb+
- Answer
-
(a) sulfide ion; 18 electrons
(b) aluminum ion, 10 electrons
(c) rubidium ion, 36 electrons
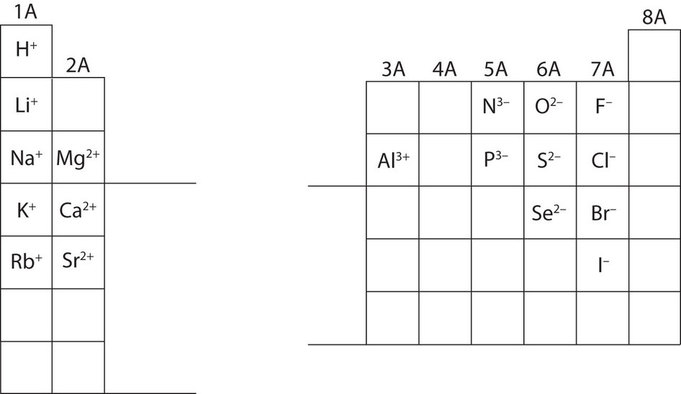
Charges of Main Group Ions
In many cases, elements that belong to the same group (vertical column) on the periodic table form ions with the same charge. Thus, the periodic table becomes a tool for remembering the charges on many ions. For example, all ions made from alkali metals, the first column on the periodic table, have a +1 charge. Ions made from alkaline earth metals, the second group on the periodic table, have a +2 charge. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a −1 charge. Figure \(\PageIndex{3}\) shows how the charge on many ions can be predicted by the location of an element on the periodic table. Note the convention of first writing the number and then the sign on a multiply charged ion. The barium cation is written Ba2+, not Ba+2.
Notice that the transition metals and most metals in groups 13 through 16 (3A through 6A) do not appear in Figure \(\PageIndex{3}\). This is because the charges on these metal ions do not follow such a regular pattern or because it is possible for the metals to form multiple ions with different charges. These cations will be discussed in the next section.
For each element, write the chemical formula and the name of the ion it forms.
- iodine
- potassium
- selenium
Solution
(a) I-, iodide ion
(b) K+, potassium ion
(c) Se2-, selenide ion
Exercise \(\PageIndex{2}\)
For each element, write the chemical formula and the name of the ion it forms.
- strontium
- bromine
- lithium
- Answer
-
(a) Sr2+, strontium ion
(b) Br-, bromide ion
(c) Li+, lithium ion

