5.2: Monotomic and Diatomic Elements
- Page ID
- 366401
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
- Name and write the symbols for the diatomic seven.
Molecular Elements
There are many substances that exist as two or more atoms connected together so strongly that they behave as a single particle. These multi-atom combinations are called molecules. A molecule is the smallest part of a substance that has the physical and chemical properties of that substance. In some respects, a molecule is similar to an atom. A molecule, however, is composed of more than one atom.
| Hydrogen | Oxygen | Nitrogen | Fluorine | Chlorine | Bromine | Iodine |
Some elements exist naturally as molecules. For example, hydrogen and oxygen exist as two-atom molecules. Other elements also exist naturally as diatomic molecules—a molecule with only two atoms (Table \(\PageIndex{1}\)). As with any molecule, these elements are labeled with a molecular formula, a formal listing of what and how many atoms are in a molecule. (Sometimes only the word formula is used, and its meaning is inferred from the context.) For example, the molecular formula for elemental hydrogen is H2, with H being the symbol for hydrogen and the subscript 2 implying that there are two atoms of this element in the molecule. Other diatomic elements have similar formulas: O2, N2, and so forth. Other elements exist as molecules—for example, sulfur normally exists as an eight-atom molecule, S8, while phosphorus exists as a four-atom molecule, P4 (Figure \(\PageIndex{1}\)). Notice that all the elements that occur as molecules are nonmetals.
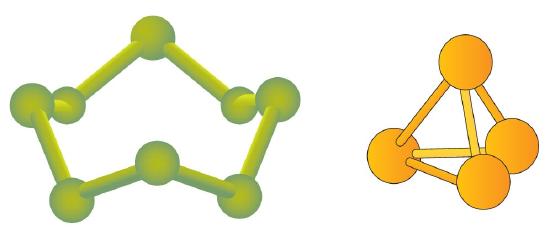
Figure \(\PageIndex{1}\) shows two examples of how molecules will be represented in this text. An atom is represented by a small ball or sphere, which generally indicates where the nucleus is in the molecule. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a molecule. This connection is called a chemical bond.
Atomic Elements
While some elements exist as molecules, most elements exist with individual atoms as their basic unit. If an element is not one of those listed above as a molecular element, assume it is an atomic element. When writing the formula for an atomic element, only the symbol for the element appears (without any subscript).
Write the formula for a pure sample of each of the elements below.
- bromine
- neon
- hydrogen
- magnesium
- nitrogen
Solution
- Br2
- Ne
- H2
- Mg
- N2
States of the Elements
Gases
It is useful to know the states of the elements at the temperatures and pressures we are likely to encounter them (around \(25^\text{o} \text{C}\) and average atmospheric pressure, also known as standard conditions). As the name of the group indicates, all of the noble gasses are gasses at these conditions. Additionally, fluorine, chlorine, oxygen, nitrogen, and hydrogen are gasses at standard conditions.
Liquids
Only two elements are liquids at standard conditions: bromine and mercury.
Solids
Most of the elements on the periodic table are solids at standard conditions. Unless an element was mentioned as being a gas or liquid above, assume it is a solid.
Indicate the state of each of the elements below at \(25^\text{o} \text{C}\) and normal atmospheric pressure.
- iodine
- carbon
- nitrogen
- bromine
- chlorine
Solution
- solid
- solid
- gas
- liquid
- gas
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Henry Agnew (UC Davis)

