6.1: Solutions and Solution Concentration
- Page ID
- 217277
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- Describe the fundamental properties of solutions
- Calculate solution concentrations using molarity and mass-mass-percent
- Perform dilution calculations using the dilution equation
In preceding sections, we focused on the composition of substances: samples of matter that contain only one type of element or compound. However, mixtures—samples of matter containing two or more substances physically combined—are more commonly encountered in nature than are pure substances. Similar to a pure substance, the relative composition of a mixture plays an important role in determining its properties. The relative amount of oxygen in a planet’s atmosphere determines its ability to sustain aerobic life. The relative amounts of iron, carbon, nickel, and other elements in steel (a mixture known as an “alloy”) determine its physical strength and resistance to corrosion. The relative amount of the active ingredient in a medicine determines its effectiveness in achieving the desired pharmacological effect. The relative amount of sugar in a beverage determines its sweetness (Figure \(\PageIndex{1}\)). In this section, we will describe one of the most common ways in which the relative compositions of mixtures may be quantified.

Figure \(\PageIndex{1}\): Sugar is one of many components in the complex mixture known as coffee. The amount of sugar in a given amount of coffee is an important determinant of the beverage’s sweetness. (credit: Jane Whitney)
Solutions
Video \(\PageIndex{1}\): An introduction to solutions from Crash Course Chemistry.
We have previously defined solutions as homogeneous mixtures, meaning that the composition of the mixture (and therefore its properties) is uniform throughout its entire volume. Solutions occur frequently in nature and have also been implemented in many forms of manmade technology. We will explore a more thorough treatment of solution properties in the chapter on solutions and colloids, but here we will introduce some of the basic properties of solutions.
The relative amount of a given solution component is known as its concentration. Often, though not always, a solution contains one component with a concentration that is significantly greater than that of all other components. This component is called the solvent and may be viewed as the medium in which the other components are dispersed, or dissolved. Solutions in which water is the solvent are, of course, very common on our planet. A solution in which water is the solvent is called an aqueous solution.
A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Solute concentrations are often described with qualitative terms such as dilute (of relatively low concentration) and concentrated (of relatively high concentration).
Video \(\PageIndex{2}\): An introduction to molarity from Crash Course Chemistry.
Concentrations of Solutions
Concentrations may be quantitatively assessed using a wide variety of measurement units, each convenient for particular applications. Some of the common concetrations measurements seen in the science labs include, parts-per-million (ppm), parts-per-billion (ppb), mass-mass-percent (m/m %), molarity (M), molality (m) and mole percent. In this unit we will use two of the more common units of measure, Molarity, and mass-mass percent.
Molarity
Molarity (M) is a useful concentration unit for many applications in chemistry. Molarity is defined as the number of moles of solute in exactly 1 liter (1 L) of the solution:
\[M=\mathrm{\dfrac{mol\: solute}{L\: solution}} \label{3.4.2}\]
The units of molarity are therefore moles per liter of solution (mol/L), abbreviated as \(M\). An aqueous solution that contains 1 mol (342 g) of sucrose in enough water to give a final volume of 1.00 L has a sucrose concentration of 1.00 mol/L or 1.00 M. In chemical notation, square brackets around the name or formula of the solute represent the molar concentration of a solute. Therefore,
\[[\rm{sucrose}] = 1.00\: M\]
is read as “the concentration of sucrose is 1.00 molar.”
Example \(\PageIndex{1}\): Calculating Molar Concentrations
A 355-mL soft drink sample contains 0.133 mol of sucrose (table sugar). What is the molar concentration of sucrose in the beverage?
Solution
Since the molar amount of solute and the volume of solution are both given, the molarity can be calculated using the definition of molarity. Per this definition, the solution volume must be converted from mL to L:
\[\begin{align*} M &=\dfrac{mol\: solute}{L\: solution} \\[5pt] &=\dfrac{0.133\:mol}{355\:mL\times \dfrac{1\:L}{1000\:mL}} \\[5pt] &= 0.375\:M \label{3.4.1} \end{align*}\]
Exercise \(\PageIndex{1}\)
A teaspoon of table sugar contains about 0.01 mol sucrose. What is the molarity of sucrose if a teaspoon of sugar has been dissolved in a cup of tea with a volume of 200 mL?
- Answer
-
0.05 M
Example \(\PageIndex{2}\): Deriving Moles and Volumes from Molar Concentrations
How much sugar (mol) is contained in a modest sip (~10 mL) of the soft drink from Example \(\PageIndex{1}\)?
Solution
In this case, we can rearrange the definition of molarity to isolate the quantity sought, moles of sugar. We then substitute the value for molarity that we derived in Example 3.4.2, 0.375 M:
\[M=\mathrm{\dfrac{mol\: solute}{L\: solution}} \label{3.4.3} \nonumber\]
\[ \begin{align*} \mathrm{mol\: solute} &= \mathrm{ M\times L\: solution} \label{3.4.4} \\[5pt] \mathrm{mol\: solute} &= \mathrm{0.375\:\dfrac{mol\: sugar}{L}\times \left(10\:mL\times \dfrac{1\:L}{1000\:mL}\right)} &= \mathrm{0.004\:mol\: sugar} \label{3.4.5} \end{align*} \]
Exercise \(\PageIndex{2}\)
What volume (mL) of the sweetened tea described in Example \(\PageIndex{1}\) contains the same amount of sugar (mol) as 10 mL of the soft drink in this example?
- Answer
-
80 mL
Example \(\PageIndex{3}\): Calculating Molar Concentrations from the Mass of Solute
Distilled white vinegar (Figure \(\PageIndex{2}\)) is a solution of acetic acid, \(CH_3CO_2H\), in water. A 0.500-L vinegar solution contains 25.2 g of acetic acid. What is the concentration of the acetic acid solution in units of molarity?

Figure \(\PageIndex{3}\): Distilled white vinegar is a solution of acetic acid in water.
Solution
As in previous examples, the definition of molarity is the primary equation used to calculate the quantity sought. In this case, the mass of solute is provided instead of its molar amount, so we must use the solute’s molar mass to obtain the amount of solute in moles:
\[\mathrm{\mathit M=\dfrac{mol\: solute}{L\: solution}=\dfrac{25.2\: g\: \ce{CH3CO2H}\times \dfrac{1\:mol\: \ce{CH3CO2H}}{60.052\: g\: \ce{CH3CO2H}}}{0.500\: L\: solution}=0.839\: \mathit M} \label{3.4.6}\]
\[\mathrm{\mathit M=\dfrac{mol\: solute}{L\: solution}=0.839\:\mathit M} \label{3.4.7}\]
\[M=\mathrm{\dfrac{0.839\:mol\: solute}{1.00\:L\: solution}} \label{3.4.8}\]
Exercise \(\PageIndex{3}\)
Calculate the molarity of 6.52 g of \(CoCl_2\) (128.9 g/mol) dissolved in an aqueous solution with a total volume of 75.0 mL.
- Answer
-
0.674 M
Example \(\PageIndex{4}\): Determining the Mass of Solute in a Given Volume of Solution
How many grams of NaCl are contained in 0.250 L of a 5.30-M solution?
Solution
The volume and molarity of the solution are specified, so the amount (mol) of solute is easily computed as demonstrated in Example \(\PageIndex{3}\):
\[M=\mathrm{\dfrac{mol\: solute}{L\: solution}} \label{3.4.9}\]
\[\mathrm{mol\: solute= \mathit M\times L\: solution} \label{3.4.10}\]
\[\mathrm{mol\: solute=5.30\:\dfrac{mol\: NaCl}{L}\times 0.250\:L=1.325\:mol\: NaCl} \label{3.4.11}\]
Finally, this molar amount is used to derive the mass of NaCl:
\[\mathrm{1.325\: mol\: NaCl\times\dfrac{58.44\:g\: NaCl}{mol\: NaCl}=77.4\:g\: NaCl} \label{3.4.12}\]
Exercise \(\PageIndex{4}\)
How many grams of \(CaCl_2\) (110.98 g/mol) are contained in 250.0 mL of a 0.200-M solution of calcium chloride?
- Answer
-
5.55 g \(CaCl_2\)
When performing calculations stepwise, as in Example \(\PageIndex{3}\), it is important to refrain from rounding any intermediate calculation results, which can lead to rounding errors in the final result. In Example \(\PageIndex{4}\), the molar amount of NaCl computed in the first step, 1.325 mol, would be properly rounded to 1.32 mol if it were to be reported; however, although the last digit (5) is not significant, it must be retained as a guard digit in the intermediate calculation. If we had not retained this guard digit, the final calculation for the mass of NaCl would have been 77.1 g, a difference of 0.3 g.
In addition to retaining a guard digit for intermediate calculations, we can also avoid rounding errors by performing computations in a single step (Example \(\PageIndex{5}\)). This eliminates intermediate steps so that only the final result is rounded.
Example \(\PageIndex{5}\): Determining the Volume of Solution
In Example \(\PageIndex{3}\), we found the typical concentration of vinegar to be 0.839 M. What volume of vinegar contains 75.6 g of acetic acid?
Solution
First, use the molar mass to calculate moles of acetic acid from the given mass:
\[\mathrm{g\: solute\times\dfrac{mol\: solute}{g\: solute}=mol\: solute} \label{3.4.13}\]
Then, use the molarity of the solution to calculate the volume of solution containing this molar amount of solute:
\[\mathrm{mol\: solute\times \dfrac{L\: solution}{mol\: solute}=L\: solution} \label{3.4.14}\]
Combining these two steps into one yields:
\[\mathrm{g\: solute\times \dfrac{mol\: solute}{g\: solute}\times \dfrac{L\: solution}{mol\: solute}=L\: solution} \label{3.4.15}\]
\[\mathrm{75.6\:g\:\ce{CH3CO2H}\left(\dfrac{mol\:\ce{CH3CO2H}}{60.05\:g}\right)\left(\dfrac{L\: solution}{0.839\:mol\:\ce{CH3CO2H}}\right)=1.50\:L\: solution} \label{3.4.16}\]
Exercise \(\PageIndex{5}\):
What volume of a 1.50-M KBr solution contains 66.0 g KBr?
- Answer
-
0.370 L
Mass-mass percent
Percentages are also commonly used to express the composition of mixtures, including solutions. The mass percentage (m/m%)of a solution component is defined as the ratio of the component’s mass to the solution’s mass, expressed as a percentage:
\[\mathrm{\% \:m/m = \dfrac{mass\: of\: solute}{mass\: of\: solution}\times100\%}\]
mass of solution = mass of solute + mass solvent
If you can measure the masses of the solute and the solution, determining the mass/mass percent is easy. Each mass must be expressed in the same units to determine the proper concentration.
Suppose that a solution was prepared by dissolving \(25.0 \: \text{g}\) of sugar into \(100.0 \: \text{g}\) of water.
The mass of the solution is
mass of solution = 25.0g sugar + 100.0g water = 125.0 g
The percent by mass would be calculated by:
\[\text{Percent by mass} = \frac{25.0 \: \text{g sugar}}{125.0 \: \text{g solution}} \times 100\% = 20.0\% \: \text{sugar}\]
Mass percentage is also referred to by similar names such as percent mass, percent weight, weight/weight percent, and other variations on this theme. The most common symbol for mass percentage is simply the percent sign, %, although more detailed symbols are often used including %mass, %weight, and (w/w)%. Use of these more detailed symbols can prevent confusion of mass percentages with other types of percentages, such as volume percentages (to be discussed later in this section).
Mass percentages are popular concentration units for consumer products. The label of a typical liquid bleach bottle (Figure 3.17) cites the concentration of its active ingredient, sodium hypochlorite (NaOCl), as being 7.4%. A 100.0-g sample of bleach would therefore contain 7.4 g of NaOCl.
Figure \(\PageIndex{4}\): Distilled white vinegar is a solution of acetic acid in water.
{6}\): Determining the Volume of Solution
A 5.0-g sample of spinal fluid contains 3.75 mg (0.00375 g) of glucose. What is the percent by mass of glucose in spinal fluid?
Solution
The spinal fluid sample contains roughly 4 mg of glucose in 5000 mg of fluid, so the mass fraction of glucose should be a bit less than one part in 1000, or about 0.1%. Substituting the given masses into the equation defining mass percentage yields:
\[\mathrm{\%\,glucose=\dfrac{3.75\;mg \;glucose \times \frac{1\;g}{1000\; mg}}{5.0\;g \;spinal\; fluid}=0.075\%}\]
The computed mass percentage agrees with our rough estimate (it’s a bit less than 0.1%).
Note that while any mass unit may be used to compute a mass percentage (mg, g, kg, oz, and so on), the same unit must be used for both the solute and the solution so that the mass units cancel, yielding a dimensionless ratio. In this case, we converted the units of solute in the numerator from mg to g to match the units in the denominator. We could just as easily have converted the denominator from g to mg instead. As long as identical mass units are used for both solute and solution, the computed mass percentage will be correct.
Exercise \(\PageIndex{6}\):
A bottle of a tile cleanser contains 135 g of HCl and 775 g of water. What is the percent by mass of HCl in this cleanser?
- Answer
-
14.8%
Example \(\PageIndex{7}\): Calculations using Mass Percentage
“Concentrated” hydrochloric acid is an aqueous solution of 37.2% HCl that is commonly used as a laboratory reagent. The density of this solution is 1.19 g/mL. What mass of HCl is contained in 0.500 L of this solution?
Solution
The HCl concentration is near 40%, so a 100-g portion of this solution would contain about 40 g of HCl. Since the solution density isn’t greatly different from that of water (1 g/mL), a reasonable estimate of the HCl mass in 500 g (0.5 L) of the solution is about five times greater than that in a 100 g portion, or \(\mathrm{5 \times 40 = 200\: g}\). To derive the mass of solute in a solution from its mass percentage, we need to know the corresponding mass of the solution. Using the solution density given, we can convert the solution’s volume to mass, and then use the given mass percentage to calculate the solute mass. This mathematical approach is outlined in this flowchart:
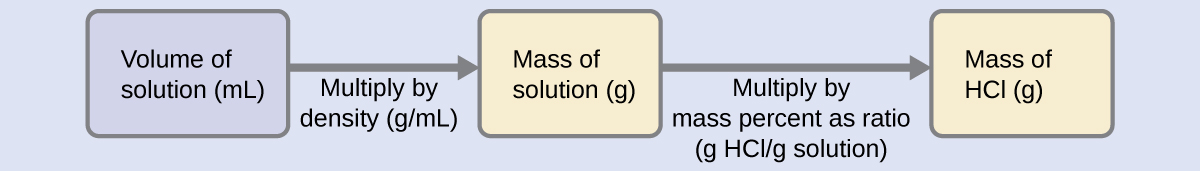
For proper unit cancellation, the 0.500-L volume is converted into 500 mL, and the mass percentage is expressed as a ratio, 37.2 g HCl/g solution:
\[ \mathrm{500\; mL\; solution \left(\dfrac{1.19\;g \;solution}{mL \;solution}\right) \left(\dfrac{37.2\;g\; HCl}{100\;g \;solution}\right)=221\;g\; HCl}\]
This mass of HCl is consistent with our rough estimate of approximately 200 g.
Exercise \(\PageIndex{7}\)
What volume of concentrated HCl solution contains 125 g of HCl?
- Answer
-
282 mL
Dilution of Solutions
Video \(\PageIndex{3}\): An introduction to dilutions from Crash Course Chemistry.
Dilution is the process whereby the concentration of a solution is lessened by the addition of solvent. For example, we might say that a glass of iced tea becomes increasingly diluted as the ice melts. The water from the melting ice increases the volume of the solvent (water) and the overall volume of the solution (iced tea), thereby reducing the relative concentrations of the solutes that give the beverage its taste (Figure \(\PageIndex{2}\)).

Figure \(\PageIndex{5}\): Both solutions contain the same mass of copper nitrate. The solution on the right is more dilute because the copper nitrate is dissolved in more solvent. (credit: Mark Ott).
Dilution is also a common means of preparing solutions of a desired concentration. By adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. For example, commercial pesticides are typically sold as solutions in which the active ingredients are far more concentrated than is appropriate for their application. Before they can be used on crops, the pesticides must be diluted. This is also a very common practice for the preparation of a number of common laboratory reagents (Figure \(\PageIndex{3}\)).

Figure \(\PageIndex{6}\): A solution of \(KMnO_4\) is prepared by mixing water with 4.74 g of KMnO4 in a flask. (credit: modification of work by Mark Ott)
A simple mathematical relationship can be used to relate the volumes and concentrations of a solution before and after the dilution process. According to the definition of molarity, the molar amount of solute in a solution is equal to the product of the solution’s molarity and its volume in liters:
\[n=ML\]
Expressions like these may be written for a solution before and after it is diluted:
\[n_1=M_1L_1\]
\[n_2=M_2L_2\]
where the subscripts “1” and “2” refer to the solution before and after the dilution, respectively. Since the dilution process does not change the amount of solute in the solution,n1 = n2. Thus, these two equations may be set equal to one another:
\[M_1L_1=M_2L_2\]
This relation is commonly referred to as the dilution equation. Although we derived this equation using molarity as the unit of concentration and liters as the unit of volume, other units of concentration and volume may be used, so long as the units properly cancel per the factor-label method. Reflecting this versatility, the dilution equation is often written in the more general form:
where \(C\) and \(V\) are concentration and volume, respectively.
Phet Simulation
Example \(\PageIndex{8}\): Determining the Concentration of a Diluted Solution
If 0.850 L of a 5.00-M solution of copper nitrate, Cu(NO3)2, is diluted to a volume of 1.80 L by the addition of water, what is the molarity of the diluted solution?
Solution
We are given the volume and concentration of a stock solution, V1 and C1, and the volume of the resultant diluted solution, V2. We need to find the concentration of the diluted solution, C2. We thus rearrange the dilution equation in order to isolate C2:
\[C_1V_1=C_2V_2\]
\[C_2=\dfrac{C_1V_1}{V_2}\]
Since the stock solution is being diluted by more than two-fold (volume is increased from 0.85 L to 1.80 L), we would expect the diluted solution’s concentration to be less than one-half 5 M. We will compare this ballpark estimate to the calculated result to check for any gross errors in computation (for example, such as an improper substitution of the given quantities). Substituting the given values for the terms on the right side of this equation yields:
\[C_2=\mathrm{\dfrac{0.850\:L\times 5.00\:\dfrac{mol}{L}}{1.80\: L}}=2.36\:M\]
This result compares well to our ballpark estimate (it’s a bit less than one-half the stock concentration, 5 M).
Exercise \(\PageIndex{8}\)
What is the concentration of the solution that results from diluting 25.0 mL of a 2.04-M solution of CH3OH to 500.0 mL?
- Answer
-
0.102 M \(CH_3OH\)
Example \(\PageIndex{9}\): Volume of a Diluted Solution
What volume of 0.12 M HBr can be prepared from 11 mL (0.011 L) of 0.45 M HBr?
Solution
We are given the volume and concentration of a stock solution, V1 and C1, and the concentration of the resultant diluted solution, C2. We need to find the volume of the diluted solution, V2. We thus rearrange the dilution equation in order to isolate V2:
\[C_1V_1=C_2V_2\]
\[V_2=\dfrac{C_1V_1}{C_2}\]
Since the diluted concentration (0.12 M) is slightly more than one-fourth the original concentration (0.45 M), we would expect the volume of the diluted solution to be roughly four times the original volume, or around 44 mL. Substituting the given values and solving for the unknown volume yields:
\[V_2=\dfrac{(0.45\:M)(0.011\: \ce L)}{(0.12\:M)}\]
\[V_2=\mathrm{0.041\:L}\]
The volume of the 0.12-M solution is 0.041 L (41 mL). The result is reasonable and compares well with our rough estimate.
Exercise \(\PageIndex{9}\)
A laboratory experiment calls for 0.125 M \(HNO_3\). What volume of 0.125 M \(HNO_3\) can be prepared from 0.250 L of 1.88 M \(HNO_3\)?
- Answer
-
3.76 L
Example \(\PageIndex{10}\): Volume of a Concentrated Solution Needed for Dilution
What volume of 1.59 M KOH is required to prepare 5.00 L of 0.100 M KOH?
Solution
We are given the concentration of a stock solution, C1, and the volume and concentration of the resultant diluted solution, V2 and C2. We need to find the volume of the stock solution, V1. We thus rearrange the dilution equation in order to isolate V1:
\[C_1V_1=C_2V_2\]
\[V_1=\dfrac{C_2V_2}{C_1}\]
Since the concentration of the diluted solution 0.100 M is roughly one-sixteenth that of the stock solution (1.59 M), we would expect the volume of the stock solution to be about one-sixteenth that of the diluted solution, or around 0.3 liters. Substituting the given values and solving for the unknown volume yields:
Thus, we would need 0.314 L of the 1.59-M solution to prepare the desired solution. This result is consistent with our rough estimate.
Exercise \(\PageIndex{10}\)
What volume of a 0.575-M solution of glucose, C6H12O6, can be prepared from 50.00 mL of a 3.00-M glucose solution?
- Answer
-
0.261 L
Summary
Solutions are homogeneous mixtures. Many solutions contain one component, called the solvent, in which other components, called solutes, are dissolved. An aqueous solution is one for which the solvent is water. The concentration of a solution is a measure of the relative amount of solute in a given amount of solution. Concentrations may be measured using various units, with one very useful unit being molarity, defined as the number of moles of solute per liter of solution. The solute concentration of a solution may be decreased by adding solvent, a process referred to as dilution. The dilution equation is a simple relation between concentrations and volumes of a solution before and after dilution.
Video \(\PageIndex{4}\): An review of calculating molarity.
Video \(\PageIndex{5}\): An review of calculating dilutions. Also, orange squash is a drink in England.
Key Equations
- \(M=\mathrm{\dfrac{mol\: solute}{L\: solution}}\)
- C1V1 = C2V2
Glossary
- aqueous solution
- solution for which water is the solvent
- concentrated
- qualitative term for a solution containing solute at a relatively high concentration
- concentration
- quantitative measure of the relative amounts of solute and solvent present in a solution
- dilute
- qualitative term for a solution containing solute at a relatively low concentration
- dilution
- process of adding solvent to a solution in order to lower the concentration of solutes
- dissolved
- describes the process by which solute components are dispersed in a solvent
- molarity (M)
- unit of concentration, defined as the number of moles of solute dissolved in 1 liter of solution
- solute
- solution component present in a concentration less than that of the solvent
- solvent
- solution component present in a concentration that is higher relative to other components
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Institute of Technology
- Fuse School, Open Educational Resource free of charge, under a Creative Commons License: Attribution-NonCommercial CC BY-NC (View License Deed: https://creativecommons.org/licenses/by-nc/4.0/)
Modified by Joshua Halpern (Howard University)

