3.1: Nuclear Chemistry and Radioactive Decay (Problems)
- Page ID
- 98692
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)PROBLEM \(\PageIndex{1}\)
Write the following isotopes in nuclide notation (e.g., " \(\ce{^{14}_6C}\) ")
- oxygen-14
- copper-70
- tantalum-175
- francium-217
- Answer a
-
\(\ce{^{14}_8O}\)
- Answer b
-
\(\ce{^{70}_29Cu}\)
- Answer c
-
\(\ce{^{175}_73Ta}\)
- Answer d
-
\(\ce{^{217}_87Fr}\)
- Click here for a video of the solution
-
PROBLEM \(\PageIndex{2}\)
For the following isotopes that have missing information, fill in the missing information to complete the notation
- \(\ce{^{34}_{14}X}\)
- \(\ce{^{36}_P}\)
- \(\ce{^{57}_{X}Mn}\)
- \(\ce{^{121}_{56}X}\)
- Answer a
-
\(\ce{^{34}_{14}Si}\)
- Answer b
-
\(\ce{^{36}_{15}P}\)
- Answer c
-
\(\ce{^{57}_{25}Mn}\)
- Answer d
-
\(\ce{^{121}_{56}Ba}\)
PROBLEM \(\PageIndex{3}\)
Write the nuclide notation, including charge if applicable, for atoms with the following characteristics:
- 25 protons, 20 neutrons, 24 electrons
- 45 protons, 24 neutrons, 43 electrons
- 53 protons, 89 neutrons, 54 electrons
- 97 protons, 146 neutrons, 97 electrons
- Answer a
-
\(\ce{^{45}_{25}Mn^{+1}}\)
- Answer b
-
\(\ce{^{69}_{45}Rh^{+2}}\)
- Answer c
-
\(\ce{^{142}_{53}I^{−1}}\)
- Answer d
-
\(\ce{^{243}_{97}Bk}\)
- Click here for a video of the solution
-
PROBLEM \(\PageIndex{4}\)
Which of the following nuclei lie within the band of stability?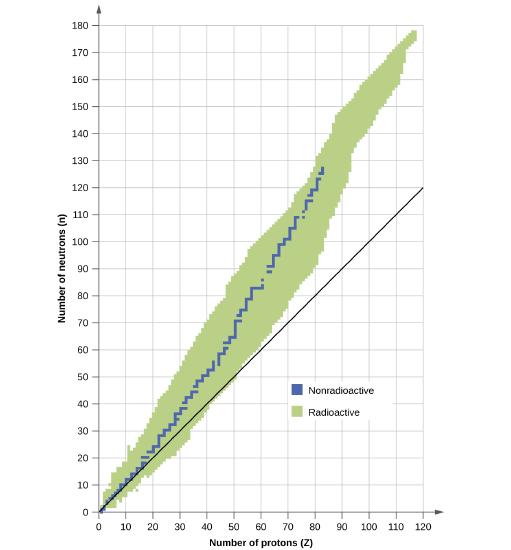
- chlorine-37
- calcium-40
- 204Bi
- 56Fe
- 206Pb
- 211Pb
- 222Rn
- carbon-14
- Answer
-
(a), (b), (c), (d), and (e)
PROBLEM \(\PageIndex{5}\)
Which of the following nuclei lie within the band of stability?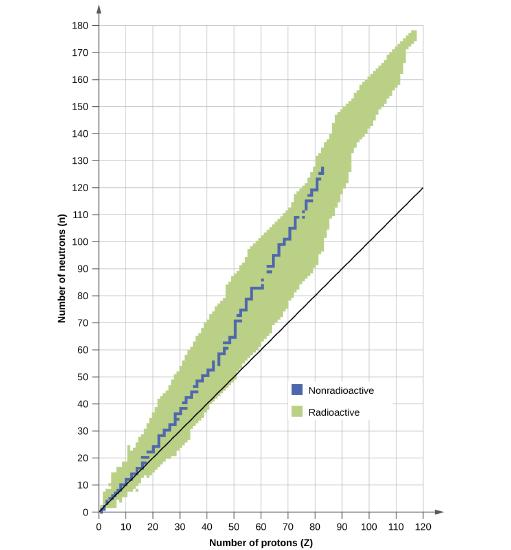
- argon-40
- oxygen-16
- 122Ba
- 58Ni
- 205Tl
- 210Tl
- 226Ra
- magnesium-24
- Answer
-
(b), (e - very close), and (h)
- Click here for a video of the solution
PROBLEM \(\PageIndex{6}\)
Write a brief description or definition of each of the following:
- nucleon
- α particle
- β particle
- positron
- γ ray
- nuclide
- mass number
- atomic number
- Answer a
-
collective term for protons and neutrons in a nucleus
- Answer b
-
(α or \(\ce{^4_2He}\) or \(\ce{^4_2α}\)) high-energy helium nucleus; a helium atom that has lost two electrons and contains two protons and two neutrons
- Answer c
-
( \(β\) or \(\ce{^0_{-1}e}\) or \(\ce{^0_{-1}β}\)) high-energy electron
- Answer d
-
antiparticle to the electron; it has identical properties to an electron, except for having the opposite (positive) charge
- Answer e
-
(γ or \(\ce{^0_0γ}\)) short wavelength, high-energy electromagnetic radiation that exhibits wave-particle duality
- Answer f
-
nucleus of a particular isotope
- Answer g
-
sum of the numbers of neutrons and protons in the nucleus of an atom
- Answer h
-
number of protons in the nucleus of an atom
PROBLEM \(\PageIndex{7}\)
Complete each of the following equations by adding the missing species:
- \(\ce{^{27}_{13}Al + ^4_2He⟶\:? + ^1_0n}\)
- \(\ce{^{239}_{94}Pu +\, ? ⟶ ^{242}_{96}Cm + ^1_0n}\)
- \(\ce{^{14}_7N + ^4_2He⟶\:? + ^1_1H}\)
- \(\ce{^{235}_{92}U⟶\:? + ^{135}_{55}Cs + 4^1_0n}\)
- Answer a
-
\(\ce{^{27}_{13}Al + ^4_2He⟶ ^{30}_{15}P + ^1_0n}\)
- Answer b
-
\(\ce{^{239}_{94}Pu + ^4_2He ⟶ ^{242}_{96}Cm + ^1_0n}\)
- Answer c
-
\(\ce{^{14}_7N + ^4_2He⟶ ^{17}_8O + ^1_1H}\)
- Answer d
-
\(\ce{^{235}_{92}U⟶ ^{96}_{37}Rb + ^{135}_{55}Cs + 4^1_0n}\)
- Click here for a video of the solution
-
PROBLEM \(\PageIndex{8}\)
Complete each of the following equations:
- \(\ce{^7_3Li +\, ?⟶2^4_2He}\)
- \(\ce{^{14}_6C⟶ ^{14}_7N +\, ?}\)
- \(\ce{^{27}_{13}Al + ^4_2He⟶\,? + ^1_0n}\)
- \(\ce{^{250}_{96}Cm ⟶\, ? + ^{98}_{38}Sr + 4^1_0n}\)
- Answer a
-
\(\ce{^7_3Li +^1_1H ⟶2^4_2He}\)
- Answer b
-
\(\ce{^{14}_6C⟶ ^{14}_7N +^0_{-1}e}\)
- Answer c
-
\(\ce{^{27}_{13}Al + ^4_2He⟶^30_15P + ^1_0n}\)
- Answer d
-
\(\ce{^{250}_{96}Cm ⟶^148_{58}Ce + ^{98}_{38}Sr + 4^1_0n}\)
PROBLEM \(\PageIndex{9}\)
Write a balanced equation for each of the following nuclear reactions:
- the production of 17O from 14N by α particle bombardment
- the production of 14C from 14N by neutron bombardment
- the production of 233Th from 232Th by neutron bombardment
- the production of 239U from 238U by \(\ce{^2_1H}\) bombardment
- Answer a
-
\(\ce{^{14}_7N + He^2 ⟶ ^{17}_8O + ^1_1H}\)
- Answer b
-
\(\ce{^{14}_7N + ^1_0n⟶ ^{14}_6N + ^1_1H}\)
- Answer c
-
\(\ce{^{232}_{90}Th + ^1_0n⟶ ^{233}_{90}Th}\)
- Answer d
-
\(\ce{^{238}_{92}U + ^2_1H⟶ ^{239}_{92}U + ^1_1H}\)
- Click here for a video of the solution
-
PROBLEM \(\PageIndex{10}\)
Technetium-99 is prepared from 98Mo. Molybdenum-98 combines with a neutron to give molybdenum-99, an unstable isotope that emits a β particle to yield an excited form of technetium-99, represented as 99Tc*. This excited nucleus relaxes to the ground state, represented as 99Tc, by emitting a γ ray. The ground state of 99Tc then emits a β particle. Write the equations for each of these nuclear reactions.
- Answer
-
\(\ce{^{98}_{42}Mo + ^1_0n \rightarrow ^{99}_{42}Mo}\)
\(\ce{^{99}_{42}Mo} \rightarrow ^0_{-1}e + ^{99}_{43}Tc^*\)
\(\ce{^{99}_{43}Tc}^* \rightarrow ^0_{0}\gamma + ^{99}_{43}Tc\)
\(\ce{^{99}_{43}Tc \rightarrow ^0_{-1}e + ^{99}_{44}Ru}\)
PROBLEM \(\PageIndex{11}\)
What changes occur to the atomic number and mass of a nucleus during each of the following decay scenarios?
- an α particle is emitted
- a β particle is emitted
- γ radiation is emitted
- a positron is emitted
- an electron is captured
- Answer a
-
Since an α particle is the same as a \(\ce{^4_2He}\) nucleus, the mass number will decrease by 4 and the atomic number will decrease by 2.
- Answer b
-
Since a β particle is the same as \(\ce{^0_{-1}e}\), the mass number will not change but the atomic number will increase by 1.
- Answer c
-
Since a γ ray has no mass (it is energy) the mass number and atomic number do not change.
- Answer d
-
A positron is the opposite of a β particle, it is \(\ce{^0_{+1}e}\), the mass number will not change but the atomic number will decrease by 1
- Answer e
-
Electron capture has the same effect on the nucleus as positron emission: The atomic number is decreased by one and the mass number does not change.
PROBLEM \(\PageIndex{12}\)
What is the change in the nucleus that results from the following decay scenarios?
- emission of a β particle
- emission of a β+ particle
- capture of an electron
- Answer a
-
conversion of a neutron to a proton: \(\ce{^1_0n ⟶ ^1_1p + ^0_{+1}e}\)
- Answer b
-
conversion of a proton to a neutron; the positron has the same mass as an electron and the same magnitude of positive charge as the electron has negative charge
when the n:p ratio of a nucleus is too low, a proton is converted into a neutron with the emission of a positron: \(\ce{^1_1p ⟶ ^1_0n + ^0_{+1}e}\)
- Answer c
-
In a proton-rich nucleus, an inner atomic electron can be absorbed. In simplest form, this changes a proton into a neutron: \(\ce{^1_1p + ^0_{-1}e ⟶ ^1_0p}\)
PROBLEM \(\PageIndex{13}\)
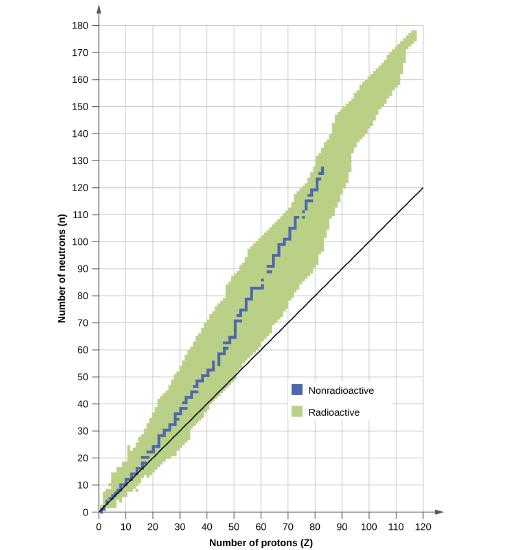 Explain how unstable heavy nuclides (atomic number > 83) may decompose to form nuclides of greater stability if
Explain how unstable heavy nuclides (atomic number > 83) may decompose to form nuclides of greater stability if
(a) they are below the band of stability and
(b) they are above the band of stability
- Answer
-
Nuclei below the band of stability will undergo positron decay, while those above the band of stability will undergo beta decay. Heavy nuclei past the band of stability will undergo alpha decay
PROBLEM \(\PageIndex{14}\)
Which of the following nuclei is most likely to decay by positron emission? Explain your choice.
- chromium-53
- manganese-51
- iron-59
- Answer
-
Manganese-51 is most likely to decay by positron emission. The n:p ratio for Cr-53 is \(\dfrac{29}{24}\) = 1.21; for Mn-51, it is \(\dfrac{26}{25}\) = 1.04; for Fe-59, it is \(\dfrac{33}{26}\) = 1.27. Positron decay occurs when the n:p ratio is low. Mn-51 has the lowest n:p ratio and therefore is most likely to decay by positron emission. Besides, \(\ce{^{53}_{24}Cr}\) is a stable isotope, and \(\ce{^{59}_{26}Fe}\) decays by beta emission.
PROBLEM \(\PageIndex{15}\)
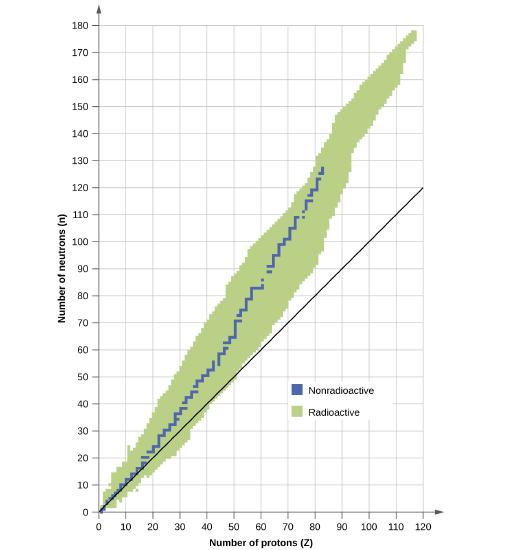 The following nuclei do not lie in the band of stability. How would they be expected to decay? Explain your answer.
The following nuclei do not lie in the band of stability. How would they be expected to decay? Explain your answer.
- \(\ce{^{34}_{15}P}\)
- \(\ce{^{239}_{92}U}\)
- \(\ce{^{38}_{20}Ca}\)
- \(\ce{^3_1H}\)
- \(\ce{^{245}_{94}Pu}\)
- Answer a
-
Above the band of stability, beta decay is expected
- Answer b
-
Beyond the band of stability, heavy nuclei undergo alpha decay
- Answer c
-
Below the band of stability, positron decay is expected
- Answer d
-
Above the band of stability, beta decay is expected
- Answer e
-
Beyond the band of stability, heavy nuclei undergo alpha decay
PROBLEM \(\PageIndex{16}\)
Write a nuclear reaction for each step in the formation of \(\ce{^{218}_{84}Po}\) from \(\ce{^{238}_{92}U}\) , which proceeds by a series of decay reactions involving the step-wise emission of α, β, β, α, α, α, α particles, in that order.
- Answer
-
\(\ce{^{238}_{92}U⟶ ^{234}_{90}Th + ^4_2He}\)
\(\ce{^{234}_{90}Th⟶ ^{234}_{91}Pa + ^0_{-1}e}\)
\(\ce{^{234}_{91}Pa⟶^{234}_{92}U + ^0_{-1}e}\)
\(\ce{^{234}_{92}U⟶ ^{230}_{90}Th + ^4_2He}\)
\(\ce{^{230}_{90}Th⟶ ^{226}_{88}Ra + ^4_2He } \)
\( \ce {^{226}_{88}Ra⟶^{222}_{86}Rn + ^4_2He}\)
\(\ce{^{222}_{86}Rn⟶^{218}_{84}Po + ^4_2He}\)
PROBLEM \(\PageIndex{17}\)
Write a nuclear reaction for each step in the formation of \(\ce{^{208}_{82}Pb}\) from \(\ce{^{228}_{90}Th}\), which proceeds by a series of decay reactions involving the step-wise emission of α, α, α, α, β, β, α particles, in that order.
- Answer
-
\(\ce{^{228}_{90}Th \rightarrow ^4_2He + ^{224}_{88}Ra}\)
\(\ce{^{224}_{88}Ra \rightarrow ^4_2He + ^{220}_{86}Rn}\)
\(\ce{^{220}_{86}Th \rightarrow ^4_2He + ^{216}_{84}Po}\)
\(\ce{^{216}_{84}Po \rightarrow ^4_2He + ^{212}_{82}Pb}\)
\(\ce{^{212}_{82}Pb \rightarrow ^0_{-1}e + ^{212}_{83}Bi}\)
\(\ce{^{212}_{83}Bi \rightarrow ^0_{-1}e + ^{212}_{84}Po}\)
\(\ce{^{212}_{84}Po \rightarrow ^4_2He + ^{208}_{82}Pb}\)
- Click here to see a video of the solution
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Adelaide Clark, Oregon Institute of Technology

