9.3: Complexation Titrations
- Page ID
- 70692
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The earliest examples of metal–ligand complexation titrations are Liebig’s determinations, in the 1850s, of cyanide and chloride using, respectively, Ag+ and Hg2+ as the titrant. Practical analytical applications of complexation titrimetry were slow to develop because many metals and ligands form a series of metal–ligand complexes. Liebig’s titration of CN– with Ag+ was successful because they form a single, stable complex of Ag(CN)2–, giving a single, easily identified end point. Other metal–ligand complexes, such as CdI42–, are not analytically useful because they form a series of metal–ligand complexes (CdI+, CdI2(aq), CdI3– and CdI42–) that produce a sequence of poorly defined end points.
Note
Recall that an acid–base titration curve for a diprotic weak acid has a single end point if its two Ka values are not sufficiently different. See Figure 9.11 for an example.
In 1945, Schwarzenbach introduced aminocarboxylic acids as multidentate ligands. The most widely used of these new ligands—ethylenediaminetetraacetic acid, or EDTA—forms strong 1:1 complexes with many metal ions. The availability of a ligand that gives a single, easily identified end point made complexation titrimetry a practical analytical method.
9.3.1 Chemistry and Properties of EDTA
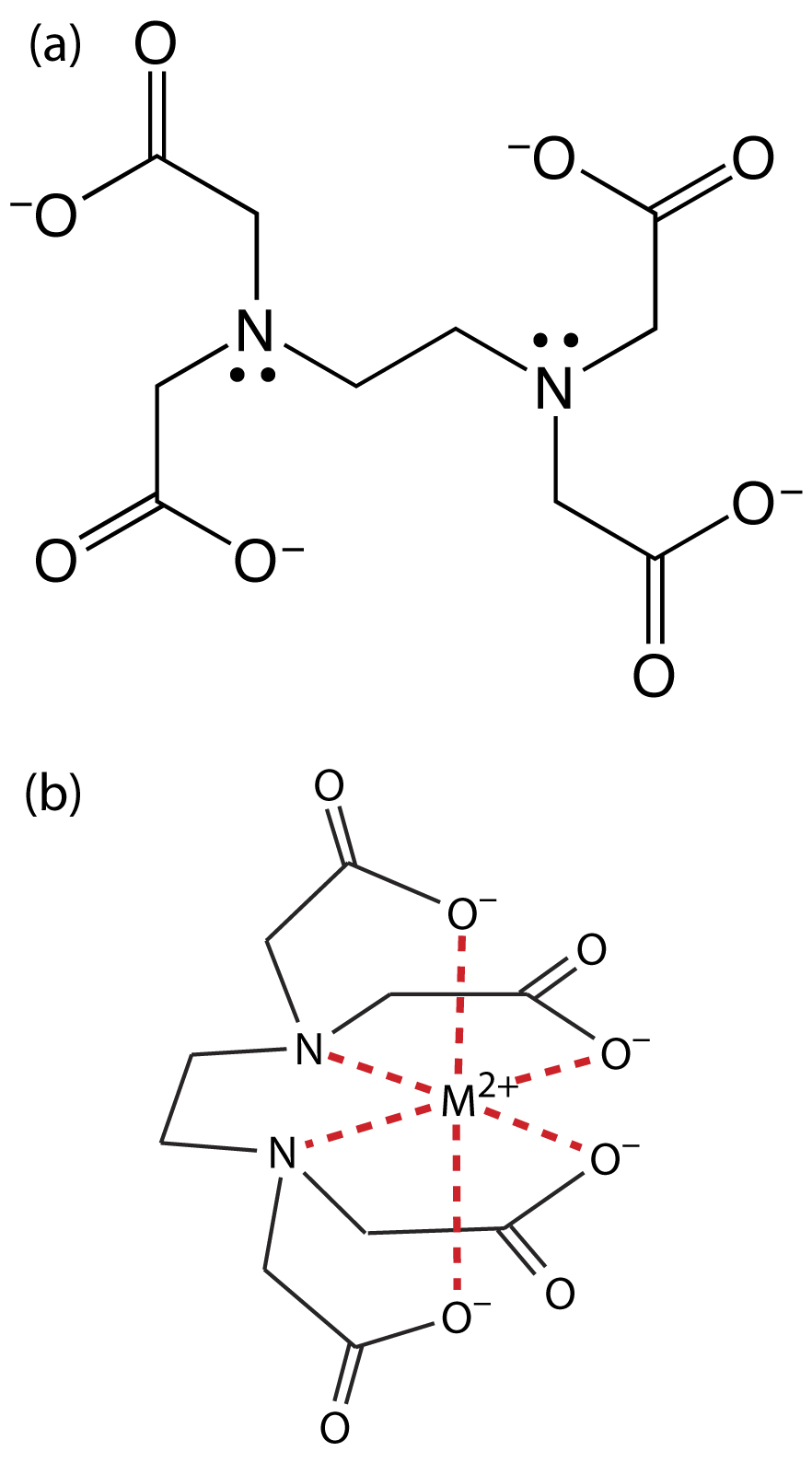
Ethylenediaminetetraacetic acid, or EDTA, is an aminocarboxylic acid. EDTA, which is shown in Figure 9.26a in its fully deprotonated form, is a Lewis acid with six binding sites—four negatively charged carboxylate groups and two tertiary amino groups—that can donate six pairs of electrons to a metal ion. The resulting metal–ligand complex, in which EDTA forms a cage-like structure around the metal ion (Figure 9.26b), is very stable. The actual number of coordination sites depends on the size of the metal ion, however, all metal–EDTA complexes have a 1:1 stoichiometry.
Figure 9.26 Structures of (a) EDTA, in its fully deprotonated form, and (b) in a six-coordinate metal–EDTA complex with a divalent metal ion.
Metal–ED TA Formation Constants
To illustrate the formation of a metal–EDTA complex, let’s consider the reaction between Cd2+ and EDTA
\[\mathrm{Cd^{2+}}(aq)+\mathrm{Y^{4-}}(aq)\rightleftharpoons \mathrm{CdY^{2-}}(aq)\tag{9.9}\]
where Y4– is a shorthand notation for the fully deprotonated form of EDTA shown in Figure 9.26a. Because the reaction’s formation constant
\[K_\textrm f=\dfrac{[\textrm{CdY}^{2-}]}{[\textrm{Cd}^{2+}][\textrm{Y}^{4-}]}=2.9\times10^{16}\tag{9.10}\]
is large, its equilibrium position lies far to the right. Formation constants for other metal–EDTA complexes are found in Table E4.
EDTA is a Weak Acid
In addition to its properties as a ligand, EDTA is also a weak acid. The fully protonated form of EDTA, H6Y2+, is a hexaprotic weak acid with successive pKa values of
|
\(\textrm pK_{\textrm a1} = 0.0\) \(\textrm pK_{\textrm a4} = 2.66\) |
\(\textrm pK_{\textrm a2} = 1.5\) \(\textrm pK_{\textrm a5} = 6.16\) |
\(\textrm pK_{\textrm a3} = 2.0\) \(\textrm pK_{\textrm a6} = 10.24\) |
The first four values are for the carboxylic acid protons and the last two values are for the ammonium protons. Figure 9.27 shows a ladder diagram for EDTA. The specific form of EDTA in reaction 9.9 is the predominate species only at pH levels greater than 10.17.
Figure 9.27 Ladder diagram for EDTA.
Conditional Metal–Ligand Formation Constants
The formation constant for CdY2– in equation 9.10 assumes that EDTA is present as Y4–. Because EDTA has many forms, when we prepare a solution of EDTA we know it total concentration, CEDTA, not the concentration of a specific form, such as Y4–. To use equation 9.10, we need to rewrite it in terms of CEDTA.
At any pH a mass balance on EDTA requires that its total concentration equal the combined concentrations of each of its forms.
\[C_\textrm{EDTA}=[\mathrm{H_6Y^{2+}}]+[\mathrm{H_5Y^+}]+[\mathrm{H_4Y}]+[\mathrm{H_3Y^-}]+[\mathrm{H_2Y^{2-}}]+[\mathrm{HY^{3-}}]+[\mathrm{Y^{4-}}]\]
To correct the formation constant for EDTA’s acid–base properties we need to calculate the fraction, αY4–, of EDTA present as Y4–.
\[\alpha_{\textrm Y^{4-}} \dfrac{[\textrm Y^{4-}]}{C_\textrm{EDTA}}\tag{9.11}\]
|
pH |
αY4– |
pH |
αY4– |
|---|---|---|---|
|
1 |
1.9×10–18 |
8 |
5.6×10–3 |
|
2 |
3.4×10–14 |
9 |
5.4×10–2 |
|
3 |
2.6×10–11 |
10 |
0.37 |
|
4 |
3.8×10–9 |
11 |
0.85 |
|
5 |
3.7×10–7 |
12 |
0.98 |
|
6 |
2.4×10–5 |
13 |
1.00 |
|
7 |
5.0×10–4 |
14 |
1.00 |
Note
Problem 9.42 from the end of chapter problems asks you to verify the values in Table 9.10 by deriving an equation for αY4-.
Table 9.10 provides values of αY4– for selected pH levels. Solving equation 9.11 for [Y4−] and substituting into equation 9.10 for the CdY2– formation constant
\[K_\textrm f =\dfrac{[\textrm{CdY}^{2-}]}{[\textrm{Cd}^{2+}]\alpha_{\textrm Y^{4-}}C_\textrm{EDTA}}\]
and rearranging gives
\[K_f'=K_f\times \alpha_{\textrm Y^{4-}}=\dfrac{[\mathrm{CdY^{2-}}]}{[\mathrm{Cd^{2+}}]C_\textrm{EDTA}}\tag{9.12}\]
where Kf´ is a pH-dependent conditional formation constant. As shown in Table 9.11, the conditional formation constant for CdY2– becomes smaller and the complex becomes less stable at more acidic pHs.
|
pH |
Kf´ |
pH |
Kf´ |
|---|---|---|---|
|
1 |
5.5 × 10–2 |
8 |
1.6 × 1014 |
|
2 |
1.0 × 103 |
9 |
1.6 × 1015 |
|
3 |
7.7 × 105 |
10 |
1.1× 1016 |
|
4 |
1.1 × 108 |
11 |
2.5 × 1016 |
|
5 |
1.1 × 1010 |
12 |
2.9 × 1016 |
|
6 |
6.8 × 1011 |
13 |
2.9 × 1016 |
|
7 |
1.5 × 1013 |
14 |
2.9 × 1016 |
EDTA Competes With Other Ligands
To maintain a constant pH during a complexation titration we usually add a buffering agent. If one of the buffer’s components is a ligand that binds Cd2+, then EDTA must compete with the ligand for Cd2+. For example, an NH4+/NH3 buffer includes NH3, which forms several stable Cd2+–NH3 complexes. Because EDTA forms a stronger complex with Cd2+ it will displace NH3, but the stability of the Cd2+–EDTA complex decreases.
We can account for the effect of an auxiliary complexing agent, such as NH3, in the same way we accounted for the effect of pH. Before adding EDTA, the mass balance on Cd2+, CCd, is
and the fraction of uncomplexed Cd2+, αCd2+, is
\[\alpha_{\textrm{Cd}^{2+}}=\dfrac{[\mathrm{Cd^{2+}}]}{C_\textrm{Cd}}\tag{9.13}\]
Note
The value of αCd2+ depends on the concentration of NH3. Contrast this with αY4-, which depends on pH.
Solving equation 9.13 for [Cd2+] and substituting into equation 9.12 gives
\[K_\textrm f' =K_\textrm f \times \alpha_{\textrm Y^{4-}} = \dfrac{[\mathrm{CdY^{2-}}]}{\alpha_\mathrm{Cd^{2+}}C_\textrm{Cd}C_\textrm{EDTA}}\]
Because the concentration of NH3 in a buffer is essentially constant, we can rewrite this equation
\[K_\textrm f''=K_\textrm f\times\alpha_\mathrm{Y^{4-}}\times\alpha_\mathrm{Cd^{2+}}=\dfrac{[\mathrm{CdY^{2-}}]}{C_\textrm{Cd}C_\textrm{EDTA}}\tag{9.14}\]
to give a conditional formation constant, Kf´´, that accounts for both pH and the auxiliary complexing agent’s concentration. Table 9.12 provides values of αM2+ for several metal ion when NH3 is the complexing agent.
|
[NH3](M) |
αCa2+ |
αCd2+ |
αCo2+ |
αCu2+ |
αMg2+ |
αNi2+ |
αZn2+ |
|---|---|---|---|---|---|---|---|
|
1 |
5.50×10–1 |
6.09×10–8 |
1.00×10–6 |
3.79×10–14 |
1.76×10–1 |
9.20×10–10 |
3.95×10–10 |
|
0.5 |
7.36×10–1 |
1.05×10–6 |
2.22×10–5 |
6.86×10–13 |
4.13×10–1 |
3.44×10–8 |
6.27×10–9 |
|
0.1 |
9.39×10–1 |
3.51×10–4 |
6.64×10–3 |
4.63×10–10 |
8.48×10–1 |
5.12×10–5 |
3.68×10–6 |
|
0.05 |
9.69×10–1 |
2.72×10–3 |
3.54×10–2 |
7.17×10–9 |
9.22×10–1 |
6.37×10–4 |
5.45×10–5 |
|
0.01 |
9.94×10–1 |
8.81×10–2 |
3.55×10–1 |
3.22×10–6 |
9.84×10–1 |
4.32×10–2 |
1.82×10–2 |
|
0.005 |
9.97×10–1 |
2.27×10–1 |
5.68×10–1 |
3.62×10–5 |
9.92×10–1 |
1.36×10–1 |
1.27×10–1 |
|
0.001 |
9.99×10–1 |
6.09×10–1 |
8.84×10–1 |
4.15×10–3 |
9.98×10–1 |
5.76×10–1 |
7.48×10–1 |
9.3.2 Complexometric EDTA Titration Curves
Now that we know something about EDTA’s chemical properties, we are ready to evaluate its usefulness as a titrant. To do so we need to know the shape of a complexometric EDTA titration curve. In section 9B we learned that an acid–base titration curve shows how the titrand’s pH changes as we add titrant. The analogous result for a complexation titration shows the change in pM, where M is the metal ion, as a function of the volume of EDTA. In this section we will learn how to calculate a titration curve using the equilibrium calculations from Chapter 6. We also will learn how to quickly sketch a good approximation of any complexation titration curve using a limited number of simple calculations.
Calculating the Titration Curve
Let’s calculate the titration curve for 50.0 mL of 5.00 × 10–3 M Cd2+ using a titrant of 0.0100 M EDTA. Furthermore, let’s assume that the titrand is buffered to a pH of 10 with a buffer that is 0.0100 M in NH3.
Note
Step 1: Calculate the conditional formation constant for the metal–EDTA complex.
Because the pH is 10, some of the EDTA is present in forms other than Y4–. In addition, EDTA must compete with NH3 for the Cd2+. To evaluate the titration curve, therefore, we first need to calculate the conditional formation constant for CdY2–. From Table 9.10 and Table 9.11 we find that αY4– is 0.35 at a pH of 10, and that αCd2+ is 0.0881 when the concentration of NH3 is 0.0100 M. Using these values, the conditional formation constant is
\[K_\textrm f''=K_\textrm f \times \alpha_\mathrm{Y^{4-}}\times\alpha_\mathrm{Cd^{2+}}=(2.9\times10^{16})(0.37)(0.0881)=9.5\times10^{14}\]
Because Kf´´ is so large, we can treat the titration reaction
\[\textrm{Cd}^{2+}(aq)+\textrm Y^{4-}(aq)\rightarrow \textrm{CdY}^{2-}(aq)\]
as if it proceeds to completion.
Note
Step 2: Calculate the volume of EDTA needed to reach the equivalence point.
The next task in calculating the titration curve is to determine the volume of EDTA needed to reach the equivalence point. At the equivalence point we know that
\[\textrm{moles EDTA = moles Cd}^{2+}\]
\[M_\textrm{EDTA}\times V_\textrm{EDTA}=M_\textrm{Cd}\times V_\textrm{Cd}\]
Substituting in known values, we find that it requires
\[V_\textrm{eq}=V_\textrm{EDTA}=\dfrac{M_\textrm{Cd}V_\textrm{Cd}}{M_\textrm{EDTA}}=\dfrac{(5.00\times10^{-3}\;\textrm M)(\textrm{50.0 mL})}{\textrm{0.0100 M}}=\textrm{25.0 mL}\]
of EDTA to reach the equivalence point.
Note
Step 3: Calculate pM values before the equivalence point by determining the concentration of unreacted metal ions.
Before the equivalence point, Cd2+ is present in excess and pCd is determined by the concentration of unreacted Cd2+. Because not all the unreacted Cd2+ is free—some is complexed with NH3—we must account for the presence of NH3. For example, after adding 5.0 mL of EDTA, the total concentration of Cd2+ is
\[\begin{align}
C_\textrm{Cd}&=\dfrac{\textrm{initial moles Cd}^{2+} - \textrm{moles EDTA added}}{\textrm{total volume}}=\dfrac{M_\textrm{Cd}V_\textrm{Cd}-M_\textrm{EDTA}V_\textrm{EDTA}}{V_\textrm{Cd}+V_\textrm{EDTA}}\\
&=\dfrac{(5.00\times10^{-3}\textrm{ M})(\textrm{50.0 mL}) - (\textrm{0.0100 M})(\textrm{5.0 mL})}{\textrm{50.0 mL + 5.0 mL}}=3.64\times10^{-3}\textrm{ M}
\end{align}\]
To calculate the concentration of free Cd2+ we use equation 9.13
\[[\mathrm{Cd^{2+}}] = \alpha_\mathrm{Cd^{2+}} \times C_\textrm{Cd} = (0.0881)(3.64\times10^{-4}\textrm{ M})=3.21\times10^{-4}\textrm{ M}\]
which gives a pCd of
\[\textrm{pCd}=-\log[\mathrm{Cd^{2+}}]=-\log(3.21\times10^{-4}) = 3.49\]
Note
Step 4: Calculate pM at the equivalence point using the conditional formation constant.
At the equivalence point all the Cd2+ initially in the titrand is now present as CdY2–. The concentration of Cd2+, therefore, is determined by the dissociation of the CdY2– complex. First, we calculate the concentration of CdY2–.
\[\begin{align}
[\mathrm{CdY^{2-}}]&=\dfrac{\textrm{initial moles Cd}^{2+}}{\textrm{total volume}}=\dfrac{M_\textrm{Cd}V_\textrm{Cd}}{V_\textrm{Cd}+V_\textrm{EDTA}}\\
&=\dfrac{(5.00\times10^{-3}\textrm{ M})(\textrm{50.0 mL})}{\textrm{50.0 mL + 25.0 mL}}=3.33\times10^{-3}\textrm{ M}
\end{align}\]
Next, we solve for the concentration of Cd2+ in equilibrium with CdY2–.
\[K_\textrm f''=\dfrac{[\mathrm{CdY^{2-}}]}{C_\textrm{Cd}C_\textrm{EDTA}}=\dfrac{3.33\times10^{-3}-x}{(x)(x)}= 9.5\times10^{14}\]
\[x=C_\textrm{Cd}=1.9\times10^{-9}\textrm{ M}\]
Note
At the equivalence point the initial moles of Cd2+ and the moles of EDTA added are equal. The total concentrations of Cd2+, CCd, and the total concentration of EDTA, CEDTA, are equal.
Once again, to find the concentration of uncomplexed Cd2+ we must account for the presence of NH3; thus
\[[\mathrm{Cd^{2+}}]=\alpha_\mathrm{Cd^{2+}}\times C_\textrm{Cd}=(0.0881)(1.9\times10^{-9}\textrm{ M}) = 1.70\times10^{-10}\textrm{ M}\]
and pCd is 9.77 at the equivalence point.
Note
Step 5: Calculate pM after the equivalence point using the conditional formation constant.
After the equivalence point, EDTA is in excess and the concentration of Cd2+ is determined by the dissociation of the CdY2– complex. First, we calculate the concentrations of CdY2– and of unreacted EDTA. For example, after adding 30.0 mL of EDTA
\[\begin{align}
[\mathrm{CdY^{2-}}]&=\dfrac{\textrm{initial moles Cd}^{2+}}{\textrm{total volume}}=\dfrac{M_\textrm{Cd}V_\textrm{Cd}}{V_\textrm{Cd}+V_\textrm{EDTA}}\\
&=\dfrac{(5.00\times10^{-3}\textrm{ M})(\textrm{50.0 mL})}{\textrm{50.0 mL + 30.0 mL}}=3.13\times10^{-3}\textrm{ M}
\end{align}\]
\[\begin{align}
C_\textrm{EDTA}&=\dfrac{M_\textrm{EDTA}V_\textrm{EDTA}-M_\textrm{Cd}V_\textrm{Cd}}{V_\textrm{Cd}+V_\textrm{EDTA}}\\
&=\dfrac{\textrm{(0.0100 M)(30.0 mL)} - (5.00\times10^{-3}\textrm{ M})(\textrm{50.0 mL})}{\textrm{50.0 mL + 30.0 mL}}\\
&=6.25\times10^{-4}\textrm{ M}
\end{align}\]
Substituting into equation 9.14 and solving for [Cd2+] gives
\[\dfrac{[\mathrm{CdY^{2-}}]}{C_\textrm{Cd}C_\textrm{EDTA}} = \dfrac{3.13\times10^{-3}\textrm{ M}}{C_\textrm{Cd}(6.25\times10^{-4}\textrm{ M})} = 9.5\times10^{14}\]
\[C_\textrm{Cd}=5.4\times10^{-15}\textrm{ M}\]
\[[\mathrm{Cd^{2+}}] = \alpha_\mathrm{Cd^{2+}} \times C_\textrm{Cd} = (0.0881)(5.4\times10^{-15}\textrm{ M}) = 4.8\times10^{-16}\textrm{ M}\]
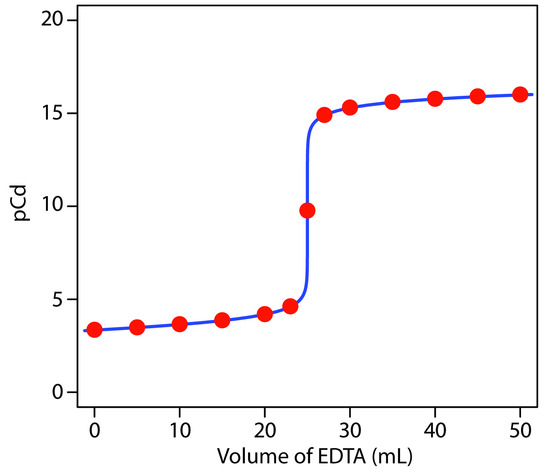
a pCd of 15.32. Table 9.13 and Figure 9.28 show additional results for this titration.
Note
After the equilibrium point we know the equilibrium concentrations of CdY2- and EDTA. We can solve for the equilibrium concentration of CCd using Kf´´ and then calculate [Cd2+] using αCd2+. Because we use the same conditional formation constant, Kf´´, for all calculations, this is the approach shown here.
There is a second method for calculating [Cd2+] after the equivalence point. Because the calculation uses only [CdY2−] and CEDTA, we can use Kf´ instead of Kf´´; thus
\[\dfrac{[\mathrm{CdY^{2-}}]}{[\mathrm{Cd^{2+}}]C_\textrm{EDTA}}=\alpha_\mathrm{Y^{4-}}\times K_\textrm f\]
\[\dfrac{3.13\times10^{-3}\textrm{ M}}{[\mathrm{Cd^{2+}}](6.25\times10^{-4}\textrm{ M})} = (0.37)(2.9\times10^{16})\]
Solving gives [Cd2+] = 4.7×10–16 M and a pCd of 15.33. We will use this approach when learning how to sketch a complexometric titration curve.
|
Volume of |
pCd |
Volume of |
pCd |
|---|---|---|---|
|
0.00 |
3.36 |
27.0 |
14.95 |
|
5.00 |
3.49 |
30.0 |
15.33 |
|
10.0 |
3.66 |
35.0 |
15.61 |
|
15.0 |
3.87 |
40.0 |
15.76 |
|
20.0 |
4.20 |
45.0 |
15.86 |
|
23.0 |
4.62 |
50.0 |
15.94 |
Figure 9.28 Titration curve for the titration of 50.0 mL of 5.00×10–3 M Cd2+ with 0.0100 M EDTA at a pH of 10 and in the presence of 0.0100 M NH3. The red points correspond to the data in Table 9.13. The blue line shows the complete titration curve.
Calculate titration curves for the titration of 50.0 mL of 5.00×10–3 M Cd2+ with 0.0100 M EDTA (a) at a pH of 10 and (b) at a pH of 7. Neither titration includes an auxiliary complexing agent. Compare your results with Figure 9.28 and comment on the effect of pH and of NH3 on the titration of Cd2+ with EDTA.
Click here to review your answer to this exercise.
Sketching an EDTA Titration Curve
To evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a reasonable approximation of the exact titration curve. In this section we demonstrate a simple method for sketching a complexation titration curve. Our goal is to sketch the titration curve quickly, using as few calculations as possible. Let’s use the titration of 50.0 mL of 5.00×10–3 M Cd2+ with 0.0100 M EDTA in the presence of 0.0100 M NH3 to illustrate our approach.
Note
This is the same example that we used in developing the calculations for a complexation titration curve. You can review the results of that calculation in Table 9.13 and Figure 9.28.
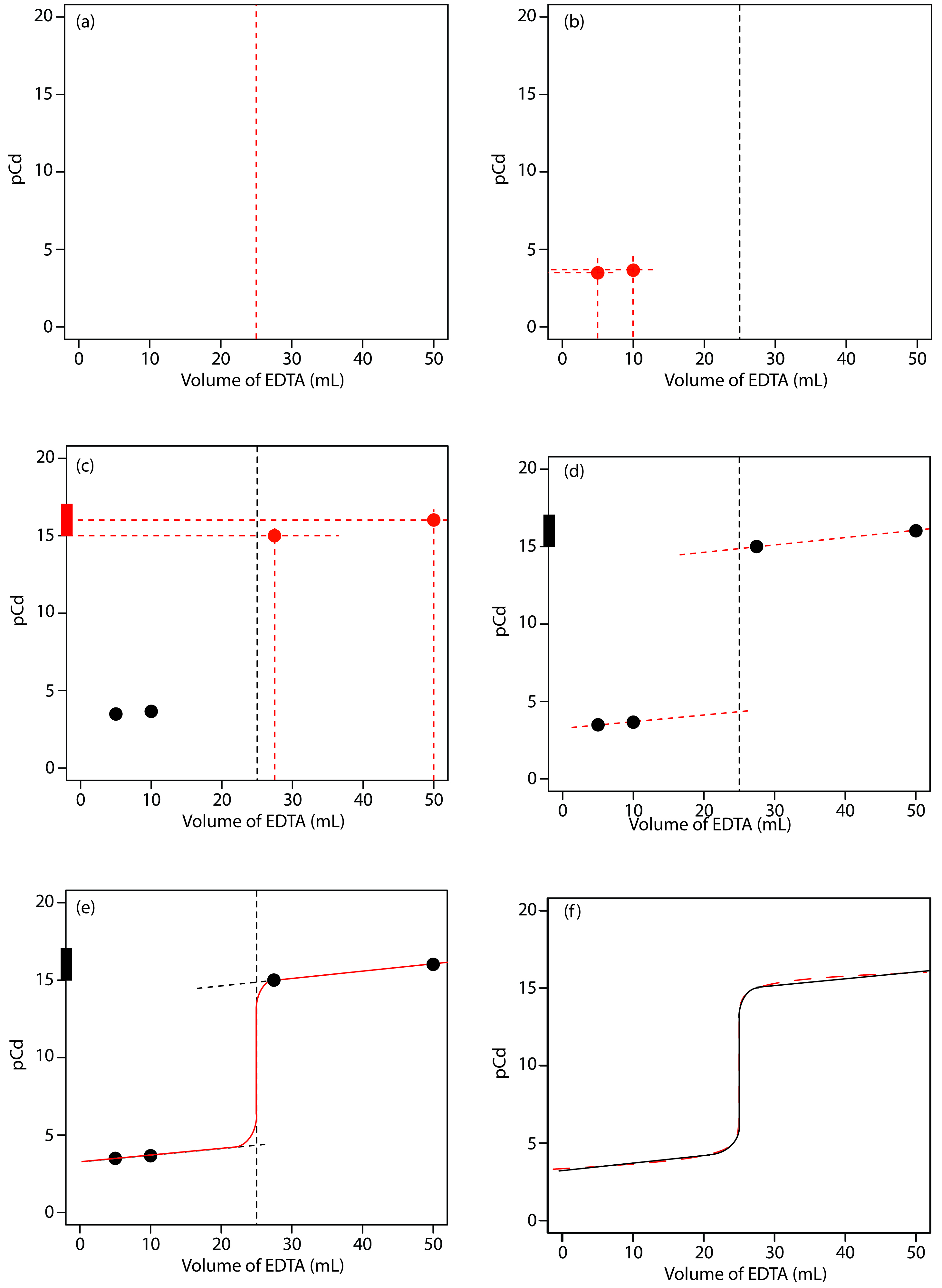
We begin by calculating the titration’s equivalence point volume, which, as we determined earlier, is 25.0 mL. Next, we draw our axes, placing pCd on the y-axis and the titrant’s volume on the x-axis. To indicate the equivalence point’s volume, we draw a vertical line corresponding to 25.0 mL of EDTA. Figure 9.29a shows the result of the first step in our sketch.
Before the equivalence point, Cd2+ is present in excess and pCd is determined by the concentration of unreacted Cd2+. Because not all the unreacted Cd2+ is free—some is complexed with NH3—we must account for the presence of NH3. The calculations are straightforward, as we saw earlier. Figure 9.29b shows the pCd after adding 5.00 mL and 10.0 mL of EDTA.
Note
See Table 9.13 for the values.
Figure 9.29 Illustrations showing the steps in sketching an approximate titration curve for the titration of 50.0 mL of 5.00 × 10–3 M Cd2+ with 0.0100 M EDTA in the presence of 0.0100 M NH3: (a) locating the equivalence point volume; (b) plotting two points before the equivalence point; (c) plotting two points after the equivalence point; (d) preliminary approximation of titration curve using straight-lines; (e) final approximation of titration curve using a smooth curve; (f) comparison of approximate titration curve (solid black line) and exact titration curve (dashed red line). See the text for additional details.
The third step in sketching our titration curve is to add two points after the equivalence point. Here the concentration of Cd2+ is controlled by the dissociation of the Cd2+–EDTA complex. Beginning with the conditional formation constant
\[K_\textrm f'=\dfrac{[\mathrm{CdY^{2-}}]}{[\mathrm{Cd^{2+}}]C_\textrm{EDTA}}=\alpha_\mathrm{Y^{4-}} \times K_\textrm f = (0.37)(2.9\times10^{16})=1.1\times10^{16}\]
we take the log of each side and rearrange, arriving at
\[\log K_\textrm f'=-\log[\mathrm{Cd^{2+}}]+\log\dfrac{[\mathrm{CdY^{2-}}]}{C_\textrm{EDTA}}\]
\[\textrm{pCd}=\log K_\textrm f'+\log\dfrac{C_\textrm{EDTA}}{[\mathrm{CdY^{2-}}]}\]
Note
See the final side comment in the previous section for an explanation of why we are ignoring the effect of NH3 on the concentration of Cd2+.
Note that after the equivalence point, the titrand’s solution is a metal–ligand complexation buffer, with pCd determined by CEDTA and [CdY2–]. The buffer is at its lower limit of pCd = logKf´ – 1 when
\[\dfrac{C_\textrm{EDTA}}{[\mathrm{CdY^{2-}}]}=\dfrac{\textrm{moles EDTA added} - \textrm{initial moles }\mathrm{Cd^{2+}}}{\textrm{initial moles }\mathrm{Cd^{2+}}}=\dfrac{1}{10}\]
Making appropriate substitutions and solving, we find that
\[\dfrac{M_\textrm{EDTA}V_\textrm{EDTA}-M_\textrm{Cd}V_\textrm{Cd}}{M_\textrm{Cd}V_\textrm{Cd}}=\dfrac{1}{10}\]
\[M_\textrm{EDTA}V_\textrm{EDTA}-M_\textrm{Cd}V_\textrm{Cd}=0.1 \times M_\textrm{Cd}V_\textrm{Cd}\]
\[V_\textrm{EDTA}=\dfrac{1.1 \times M_\textrm{Cd}V_\textrm{Cd}}{M_\textrm{EDTA}}=1.1\times V_\textrm{eq}\]
Thus, when the titration reaches 110% of the equivalence point volume, pCd is logKf´ – 1. A similar calculation should convince you that pCd = logKf´ when the volume of EDTA is 2×Veq.
Note
Our derivation here is general and applies to any complexation titration using EDTA as a titrant.
Figure 9.29c shows the third step in our sketch. First, we add a ladder diagram for the CdY2– complex, including its buffer range, using its logKf´ value of 16.04. Next, we add points representing pCd at 110% of Veq (a pCd of 15.04 at 27.5 mL) and at 200% of Veq (a pCd of 16.04 at 50.0 mL).
Next, we draw a straight line through each pair of points, extending the line through the vertical line representing the equivalence point’s volume (Figure 9.29d). Finally, we complete our sketch by drawing a smooth curve that connects the three straight-line segments (Figure 9.29e). A comparison of our sketch to the exact titration curve (Figure 9.29f) shows that they are in close agreement.
Sketch titration curves for the titration of 50.0 mL of 5.00×10–3 M Cd2+ with 0.0100 M EDTA (a) at a pH of 10 and (b) at a pH of 7. Compare your sketches to the calculated titration curves from Practice Exercise 9.12.
Click here to review your answer to this exercise.
9.3.3 Selecting and Evaluating the End point
The equivalence point of a complexation titration occurs when we react stoichiometrically equivalent amounts of titrand and titrant. As is the case with acid–base titrations, we estimate the equivalence point of a complexation titration using an experimental end point. A variety of methods are available for locating the end point, including indicators and sensors that respond to a change in the solution conditions.
Finding the End point with an Indicator
Most indicators for complexation titrations are organic dyes—known as metallochromic indicators—that form stable complexes with metal ions. The indicator, Inm–, is added to the titrand’s solution where it forms a stable complex with the metal ion, MInn–. As we add EDTA it reacts first with free metal ions, and then displaces the indicator from MInn–.
\[\textrm{MIn}^{n-}+\textrm Y^{4-}\rightarrow\textrm{MY}^{2-}+\textrm{In}^{m-}\]
If MInn– and Inm– have different colors, then the change in color signals the end point.
The accuracy of an indicator’s end point depends on the strength of the metal–indicator complex relative to that of the metal–EDTA complex. If the metal–indicator complex is too strong, the change in color occurs after the equivalence point. If the metal–indicator complex is too weak, however, the end point occurs before we reach the equivalence point.
Most metallochromic indicators also are weak acids. One consequence of this is that the conditional formation constant for the metal–indicator complex depends on the titrand’s pH. This provides some control over an indicator’s titration error because we can adjust the strength of a metal–indicator complex by adjusted the pH at which we carry out the titration. Unfortunately, because the indicator is a weak acid, the color of the uncomplexed indicator also changes with pH. Figure 9.30, for example, shows the color of the indicator calmagite as a function of pH and pMg, where H2In–, HIn2–, and In3– are different forms of the uncomplexed indicator, and MgIn– is the Mg2+–calmagite complex. Because the color of calmagite’s metal–indicator complex is red, its use as a metallochromic indicator has a practical pH range of approximately 8.5–11 where the uncomplexed indicator, HIn2–, has a blue color.
Note
Figure 9.30 is essentially a two-variable ladder diagram. The solid lines are equivalent to a step on a conventional ladder diagram, indicating conditions where two (or three) species are equal in concentration.
Figure 9.30 (a) Predominance diagram for the metallochromic indicator calmagite showing the most important form and color of calmagite as a function of pH and pMg, where H2In–, HIn2–, and In3– are uncomplexed forms of calmagite, and MgIn– is its complex with Mg2+. Conditions to the right of the dashed line, where Mg2+ precipitates as Mg(OH)2, are not analytically useful for a complexation titration. A red to blue end point is possible if we maintain the titrand’s pH in the range 8.5–11. (b) Diagram showing the relationship between the concentration of Mg2+ (as pMg) and the indicator’s color. The ladder diagram defines pMg values where MgIn– and HIn– are predominate species. The indicator changes color when pMg is between logKf – 1 and logKf + 1.
Table 9.14 provides examples of metallochromic indicators and the metal ions and pH conditions for which they are useful. Even if a suitable indicator does not exist, it is often possible to complete an EDTA titration by introducing a small amount of a secondary metal–EDTA complex, if the secondary metal ion forms a stronger complex with the indicator and a weaker complex with EDTA than the analyte. For example, calmagite gives poor end points when titrating Ca2+ with EDTA. Adding a small amount of Mg2+–EDTA to the titrand gives a sharper end point. Because Ca2+ forms a stronger complex with EDTA, it displaces Mg2+, which then forms the red-colored Mg2+–calmagite complex. At the titration’s end point, EDTA displaces Mg2+ from the Mg2+–calmagite complex, signaling the end point by the presence of the uncomplexed indicator’s blue form.
|
Indicator |
pH Range |
Metal Ionsa |
|---|---|---|
|
calmagite |
8.5–11 |
Ba, Ca, Mg, Zn |
|
eriochrome Black T |
7.5–10.5 |
Ba, Ca, Mg, Zn |
|
eriochrome Blue Black R |
8–12 |
Ca, Mg, Zn, Cu |
|
murexide |
6–13 |
Ca, Ni, Cu |
|
PAN |
2–11 |
Cd, Cu, Zn |
|
salicylic acid |
2–3 |
Fe |
a metal ions in italic font have poor end points
Finding the End point by Monitoring Absorbance
Note
Two other methods for finding the end point of a complexation titration are a thermometric titration, in which we monitor the titrand’s temperature as we add the titrant, and a potentiometric titration in which we use an ion selective electrode to monitor the metal ion’s concentration as we add the titrant. The experimental approach is essentially identical to that described earlier for an acid–base titration, to which you may refer.
See Chapter 11 for more details about ion selective electrodes.
An important limitation when using an indicator is that we must be able to see the indicator’s change in color at the end point. This may be difficult if the solution is already colored. For example, when titrating Cu2+ with EDTA, ammonia is used to adjust the titrand’s pH. The intensely colored Cu(NH3)42+ complex obscures the indicator’s color, making an accurate determination of the end point difficult. Other absorbing species present within the sample matrix may also interfere. This is often a problem when analyzing clinical samples, such as blood, or environmental samples, such as natural waters.
If at least one species in a complexation titration absorbs electromagnetic radiation, we can identify the end point by monitoring the titrand’s absorbance at a carefully selected wavelength. For example, we can identify the end point for a titration of Cu2+ with EDTA, in the presence of NH3 by monitoring the titrand’s absorbance at a wavelength of 745 nm, where the Cu(NH3)42+ complex absorbs strongly. At the beginning of the titration the absorbance is at a maximum. As we add EDTA, however, the reaction
\[\mathrm{Cu(NH_3)_4^{2+}}(aq)+\textrm Y^{4-}(aq)\rightarrow\textrm{CuY}^{2-}(aq)+4\mathrm{NH_3}(aq)\]
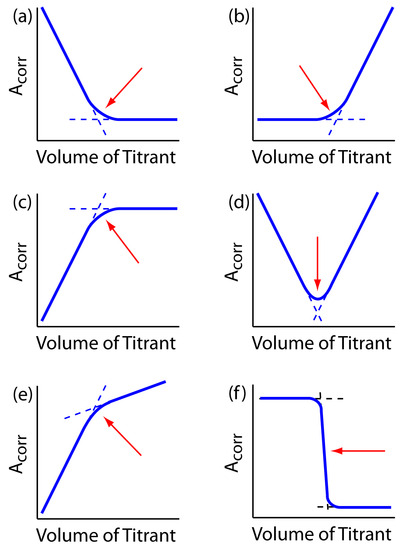
decreases the concentration of Cu(NH3)42+ and decreases the absorbance until we reach the equivalence point. After the equivalence point the absorbance remains essentially unchanged. The resulting spectrophotometric titration curve is shown in Figure 9.31a. Note that the titration curve’s y-axis is not the actual absorbance, A, but a corrected absorbance, Acorr
\[A_\textrm{corr}=A\times\dfrac{V_\textrm{EDTA}+V_\textrm{Cu}}{V_\textrm{Cu}}\]
where VEDTA and VCu are, respectively, the volumes of EDTA and Cu. Correcting the absorbance for the titrand’s dilution ensures that the spectrophotometric titration curve consists of linear segments that we can extrapolate to find the end point. Other common spectrophotometric titration curves are shown in Figures 9.31b-f.
Figure 9.31 Examples of spectrophotometric titration curves: (a) only the titrand absorbs; (b) only the titrant absorbs; (c) only the product of the titration reaction absorbs; (d) both the titrand and the titrant absorb; (e) both the titration reaction’s product and the titrant absorb; (f) only the indicator absorbs. The red arrows indicate the end points for each titration curve.
Note
The best way to appreciate the theoretical and practical details discussed in this section is to carefully examine a typical complexation titrimetric method. Although each method is unique, the following description of the determination of the hardness of water provides an instructive example of a typical procedure. The description here is based on Method 2340C as published in Standard Methods for the Examination of Water and Wastewater, 20th Ed., American Public Health Association: Washington, D. C., 1998.
Determination of Hardness of Water and Wastewater
Description of the Method
The operational definition of water hardness is the total concentration of cations in a sample capable of forming insoluble complexes with soap. Although most divalent and trivalent metal ions contribute to hardness, the most important are Ca2+ and Mg2+. Hardness is determined by titrating with EDTA at a buffered pH of 10. Calmagite is used as an indicator. Hardness is reported as mg CaCO3/L.
Procedure
Select a volume of sample requiring less than 15 mL of titrant to keep the analysis time under 5 minutes and, if necessary, dilute the sample to 50 mL with distilled water. Adjust the sample’s pH by adding 1–2 mL of a pH 10 buffer containing a small amount of Mg2+–EDTA. Add 1–2 drops of indicator and titrate with a standard solution of EDTA until the red-to-blue end point is reached (Figure 9.32).
Questions
1. Why is the sample buffered to a pH of 10? What problems might you expect at a higher pH or a lower pH?
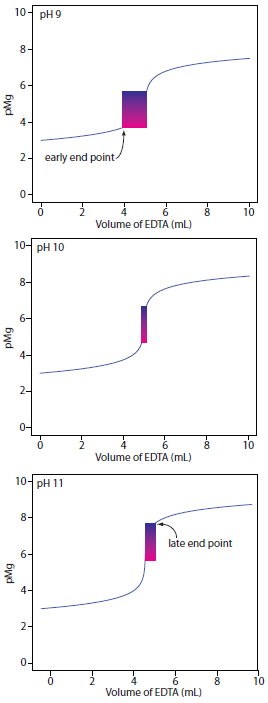
Of the cations contributing to hardness, Mg2+ forms the weakest complex with EDTA and is the last cation to be titrated. Calmagite is a useful indicator because it gives a distinct end point when titrating Mg2+. Because of calmagite’s acid–base properties, the range of pMg values over which the indicator changes color is pH–dependent (Figure 9.30). Figure 9.33 shows the titration curve for a 50-mL solution of 10–3 M Mg2+ with 10–2 M EDTA at pHs of 9, 10, and 11. Superimposed on each titration curve is the range of conditions for which the average analyst will observe the end point. At a pH of 9 an early end point is possible, leading to a negative determinate error. A late end point and a positive determinate error are possible if we use a pH of 11.
2. Why is a small amount of the Mg2+–EDTA complex added to the buffer?
The titration’s end point is signaled by the indicator calmagite. The indicator’s end point with Mg2+ is distinct, but its change in color when titrating Ca2+ does not provide a good end point. If the sample does not contain any Mg2+ as a source of hardness, then the titration’s end point is poorly defined, leading to inaccurate and imprecise results.
Adding a small amount of Mg2+–EDTA to the buffer ensures that the titrand includes at least some Mg2+. Because Ca2+ forms a stronger complex with EDTA, it displaces Mg2+ from the Mg2+–EDTA complex, freeing the Mg2+ to bind with the indicator. This displacement is stoichiometric, so the total concentration of hardness cations remains unchanged. The displacement by EDTA of Mg2+ from the Mg2+–indicator complex signals the titration’s end point.
3. Why does the procedure specify that the titration take no longer than 5 minutes?
A time limitation suggests that there is a kinetically controlled interference, possibly arising from a competing chemical reaction. In this case the interference is the possible precipitation of CaCO3 at a pH of 10.
Figure 9.32 End point for the titration of hardness with EDTA using calmagite as an indicator; the indicator is: (a) red prior to the end point due to the presence of the Mg2+–indicator complex; (b) purple at the titration’s end point; and (c) blue after the end point due to the presence of uncomplexed indicator.
Figure 9.33 Titration curves for 50 mL of 10–3 M Mg2+ with 10–3 M EDTA at pHs 9, 10, and 11 using calmagite as an indicator. The range of pMg and volume of EDTA over which the indicator changes color is shown for each titration curve.
9.3.4 Quantitative Applications
Although many quantitative applications of complexation titrimetry have been replaced by other analytical methods, a few important applications continue to be relevant. In the section we review the general application of complexation titrimetry with an emphasis on applications from the analysis of water and wastewater. First, however, we discuss the selection and standardization of complexation titrants.
Selection and Standardization of Titrants
EDTA is a versatile titrant that can be used to analyze virtually all metal ions. Although EDTA is the usual titrant when the titrand is a metal ion, it cannot be used to titrate anions. In the later case, Ag+ or Hg2+ are suitable titrants.
Solutions of EDTA are prepared from its soluble disodium salt, Na2H2Y•2H2O and standardized by titrating against a solution made from the primary standard CaCO3. Solutions of Ag+ and Hg2+ are prepared using AgNO3 and Hg(NO3)2, both of which are secondary standards. Standardization is accomplished by titrating against a solution prepared from primary standard grade NaCl.
Inorganic Analysis
Complexation titrimetry continues to be listed as a standard method for the determination of hardness, Ca2+, CN–, and Cl– in waters and wastewaters. The evaluation of hardness was described earlier in Representative Method 9.2. The determination of Ca2+ is complicated by the presence of Mg2+, which also reacts with EDTA. To prevent an interference the pH is adjusted to 12–13, precipitating Mg2+ as Mg(OH)2. Titrating with EDTA using murexide or Eriochrome Blue Black R as the indicator gives the concentration of Ca2+.
Cyanide is determined at concentrations greater than 1 mg/L by making the sample alkaline with NaOH and titrating with a standard solution of AgNO3, forming the soluble Ag(CN)2– complex. The end point is determined using p-dimethylaminobenzalrhodamine as an indicator, with the solution turning from a yellow to a salmon color in the presence of excess Ag+.
Chloride is determined by titrating with Hg(NO3)2, forming HgCl2(aq). The sample is acidified to a pH of 2.3–3.8 and diphenylcarbazone, which forms a colored complex with excess Hg2+, serves as the indicator. A pH indicator—xylene cyanol FF—is added to ensure that the pH is within the desired range. The initial solution is a greenish blue, and the titration is carried out to a purple end point.
Quantitative Calculations
The quantitative relationship between the titrand and the titrant is determined by the stoichiometry of the titration reaction. For a titration using EDTA, the stoichiometry is always 1:1.
The concentration of a solution of EDTA was determined by standardizing against a solution of Ca2+ prepared using a primary standard of CaCO3. A 0.4071-g sample of CaCO3 was transferred to a 500-mL volumetric flask, dissolved using a minimum of 6 M HCl, and diluted to volume. After transferring a 50.00-mL portion of this solution to a 250-mL Erlenmeyer flask, the pH was adjusted by adding 5 mL of a pH 10 NH3–NH4Cl buffer containing a small amount of Mg2+–EDTA. After adding calmagite as an indicator, the solution was titrated with the EDTA, requiring 42.63 mL to reach the end point. Report the molar concentration of EDTA in the titrant.
(Note that in this example, the analyte is the titrant.)
Solution
The primary standard of Ca2+ has a concentration of
\[\dfrac{0.4071\textrm{ g CaCO}_3}{\textrm{0.5000 L}}\times\dfrac{\textrm{1 mol Ca}^{2+}}{100.09\textrm{ g CaCO}_3}=8.135\times10^{-3}\textrm{ M Ca}^{2+}\]
The moles of Ca2+ in the titrand is
\[8.135\times10^{-3}\textrm{ M Ca}^{2+}\times0.05000\textrm{ L Ca}^{2+} = 4.068\times10^{-4}\textrm{ mol Ca}^{2+}\]
which means that 4.068×10–4 moles of EDTA are used in the titration. The molarity of EDTA in the titrant is
\[\mathrm{\dfrac{4.068\times10^{-4}\;mol\;EDTA}{0.04263\;L\;EDTA} = 9.543\times10^{-3}\;M\;EDTA}\]
A 100.0-mL sample is analyzed for hardness using the procedure outlined in Representative Method 9.2, requiring 23.63 mL of 0.0109 M EDTA. Report the sample’s hardness as mg CaCO3/L.
Click here to review your answer to this exercise.
As shown in the following example, we can easily extended this calculation to complexation reactions using other titrants.
The concentration of Cl– in a 100.0-mL sample of water from a freshwater aquifer was tested for the encroachment of sea water by titrating with 0.0516 M Hg(NO3)2. The sample was acidified and titrated to the diphenylcarbazone end point, requiring 6.18 mL of the titrant. Report the concentration of Cl–, in mg/L, in the aquifer.
Solution
The reaction between Cl– and Hg2+ produces a metal–ligand complex of HgCl2(aq). Each mole of Hg2+ reacts with 2 moles of Cl–; thus
\[\mathrm{\dfrac{0.0516\;mol\;Hg(NO_3)_2}{L}\times0.00618\;L\;Hg(NO_3)_2\times\dfrac{2\;mol\;Cl^-}{mol\;Hg(NO_3)_2}\times\dfrac{35.453\;g\;Cl^-}{mol\;Cl^-}=0.0226\;g\;Cl^-}\]
are in the sample. The concentration of Cl– in the sample is
\[\dfrac{0.0226\textrm{ g Cl}^-}{0.1000\textrm{ L}}\times\dfrac{\textrm{1000 mg}}{\textrm g}=226\textrm{ mg/L}\]
A 0.4482-g sample of impure NaCN is titrated with 0.1018 M AgNO3, requiring 39.68 mL to reach the end point. Report the purity of the sample as %w/w NaCN.
Click here to review your answer to this exercise.
Finally, complex titrations involving multiple analytes or back titrations are possible.
An alloy of chromel containing Ni, Fe, and Cr was analyzed by a complexation titration using EDTA as the titrant. A 0.7176-g sample of the alloy was dissolved in HNO3 and diluted to 250 mL in a volumetric flask. A 50.00-mL aliquot of the sample, treated with pyrophosphate to mask the Fe and Cr, required 26.14 mL of 0.05831 M EDTA to reach the murexide end point. A second 50.00-mL aliquot was treated with hexamethylenetetramine to mask the Cr. Titrating with 0.05831 M EDTA required 35.43 mL to reach the murexide end point. Finally, a third 50.00-mL aliquot was treated with 50.00 mL of 0.05831 M EDTA, and back titrated to the murexide end point with 6.21 mL of 0.06316 M Cu2+. Report the weight percents of Ni, Fe, and Cr in the alloy.
Solution
The stoichiometry between EDTA and each metal ion is 1:1. For each of the three titrations, therefore, we can easily equate the moles of EDTA to the moles of metal ions that are titrated.
Titration 1: moles Ni = moles EDTA
Titration 2: moles Ni + moles Fe = moles EDTA
Titration 3: moles Ni + moles Fe + moles Cr + moles Cu = moles EDTA
We can use the first titration to determine the moles of Ni in our 50.00-mL portion of the dissolved alloy. The titration uses
\[\mathrm{\dfrac{0.05831\;mol\;EDTA}{L}\times 0.02614\;L\;EDTA=1.524\times10^{-3}\;mol\;EDTA}\]
which means the sample contains 1.524×10–3 mol Ni.
Having determined the moles of EDTA reacting with Ni, we can use the second titration to determine the amount of Fe in the sample. The second titration uses
\[\mathrm{\dfrac{0.05831\;mol\;EDTA}{L}\times0.03543\;L\;EDTA=2.066\times10^{-3}\;mol\;EDTA}\]
of which 1.524×10–3 mol are used to titrate Ni. This leaves 5.42×10–4 mol of EDTA to react with Fe; thus, the sample contains 5.42×10–4 mol of Fe.
Finally, we can use the third titration to determine the amount of Cr in the alloy. The third titration uses
\[\mathrm{\dfrac{0.05831\;mol\;EDTA}{L}\times0.05000\;L\;EDTA=2.916\times10^{-3}\;mol\;EDTA}\]
of which 1.524×10–3 mol are used to titrate Ni and 5.42×10–4 mol are used to titrate Fe. This leaves 8.50×10–4 mol of EDTA to react with Cu and Cr. The amount of EDTA reacting with Cu is
\[\mathrm{\dfrac{0.06316\;mol\;Cu^{2+}}{L}\times0.00621\;L\;Cu^{2+}\times\dfrac{1\;mol\;EDTA}{mol\;Cu^{2+}}=3.92\times10^{-4}\;mol\;EDTA}\]
leaving 4.58×10–4 mol of EDTA to react with Cr. The sample, therefore, contains 4.58×10–4 mol of Cr.
Having determined the moles of Ni, Fe, and Cr in a 50.00-mL portion of the dissolved alloy, we can calculate the %w/w of each analyte in the alloy.
\[\mathrm{\dfrac{1.524\times10^{-3}\;mol\;Ni}{50.00\;mL}\times250.0\;mL\times\dfrac{58.69\;g\;Ni}{mol\;Ni}=0.4472\;g\;Ni}\]
\[\mathrm{\dfrac{0.4472\;g\;Ni}{0.7176\;g\;sample}\times100=62.32\%\;w/w\;Ni}\]
\[\mathrm{\dfrac{5.42\times10^{-4}\;mol\;Fe}{50.00\;mL}\times250.0\;mL\times\dfrac{55.847\;g\;Fe}{mol\;Fe}=0.151\;g\;Fe}\]
\[\mathrm{\dfrac{0.151\;g\;Fe}{0.7176\;g\;sample}\times100=21.0\%\;w/w\;Fe}\]
\[\mathrm{\dfrac{4.58\times10^{-4}\;mol\;Cr}{50.00\;mL}\times250.0\;mL\times\dfrac{51.996\;g\;Cr}{mol\;Cr}=0.119\;g\;Cr}\]
\[\mathrm{\dfrac{0.119\;g\;Cr}{0.7176\;g\;sample}\times100=16.6\%\;w/w\;Fe}\]
A indirect complexation titration with EDTA can be used to determine the concentration of sulfate, SO42–, in a sample. A 0.1557-g sample is dissolved in water, any sulfate present is precipitated as BaSO4 by adding Ba(NO3)2. After filtering and rinsing the precipitate, it is dissolved in 25.00 mL of 0.02011 M EDTA. The excess EDTA is then titrated with 0.01113 M Mg2+, requiring 4.23 mL to reach the end point. Calculate the %w/w Na2SO4 in the sample.
Click here to review your answer to this exercise.
9.3.5 Evaluation of Complexation Titrimetry
The scale of operations, accuracy, precision, sensitivity, time, and cost of a complexation titration are similar to those described earlier for acid–base titrations. Complexation titrations, however, are more selective. Although EDTA forms strong complexes with most metal ion, by carefully controlling the titrand’s pH we can analyze samples containing two or more analytes. The reason we can use pH to provide selectivity is shown in Figure 9.34a. A titration of Ca2+ at a pH of 9 gives a distinct break in the titration curve because the conditional formation constant for CaY2– of 2.6 × 109 is large enough to ensure that the reaction of Ca2+ and EDTA goes to completion. At a pH of 3, however, the conditional formation constant of 1.23 is so small that very little Ca2+ reacts with the EDTA.
Suppose we need to analyze a mixture of Ni2+ and Ca2+. Both analytes react with EDTA, but their conditional formation constants differ significantly. If we adjust the pH to 3 we can titrate Ni2+ with EDTA without titrating Ca2+ (Figure 9.34b). When the titration is complete, we adjust the titrand’s pH to 9 and titrate the Ca2+ with EDTA.
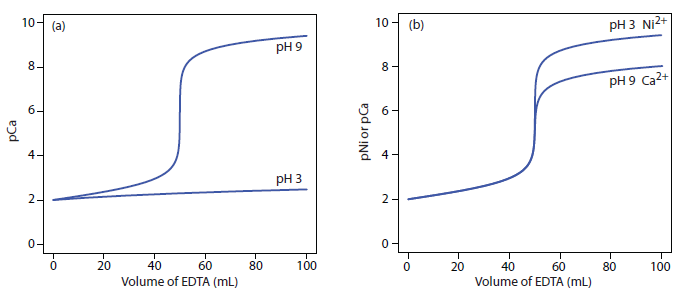
Figure 9.34 Titration curves illustrating how we can use the titrand’s pH to control EDTA’s selectivity. (a) Titration of 50.0 mL of 0.010 M Ca2+ at a pH of 3 and a pH of 9 using 0.010 M EDTA. At a pH of 3 the CaY2– complex is too weak to successfully titrate. (b) Titration of a 50.0 mL mixture of 0.010 M Ca2+ and 0.010 M Ni2+ at a pH of 3 and a pH of 9 using 0.010 M EDTA. At a pH of 3 EDTA reacts only with Ni2+. When the titration is complete, raising the pH to 9 allows for the titration of Ca2+.
A spectrophotometric titration is a particularly useful approach for analyzing a mixture of analytes. For example, as shown in Figure 9.35, we can determine the concentration of a two metal ions if there is a difference between the absorbance of the two metal-ligand complexes.
Figure 9.35 Spectrophotometric titration curve for the complexation titration of a mixture of two analytes. The red arrows indicate the end points for each analyte.