2: Physical principles underlying chemistry
- Page ID
- 16223
Research in chemistry proceeds along two directions: Experimental and theoretical. Experimental chemistry is the science of observation. We pose a question to nature, carry out the corresponding experiment, and observe the outcome. What happens if we mix two substances? If we blast a molecule with a laser, what will it do? If we introduce a biomimetic into a living organism, how will it respond? Experiments produce data that relate to each individual observation. Theory, on the other hand, is concerned with building general frameworks for placing large numbers of individual observations into a rational order. It does so by building mathematical models, deducing general laws, and using these to predict the outcomes of future experiments. The tools of theory are usually mathematics and physics applied to chemical problems. Without theory, experimental science is just a vast catalog of unrationalized observations. Without experiment, theory is simply a long list of conjectures with no verification or falsification.
Keeping this juxtaposition in mind, we briefly review the basic laws of chemical transformations, keeping in mind their historical origins and the observations out of which they grew.
Fundamental laws of chemical reactions and chemical equations
1. The law of conservation of mass (Lavoisier, 18th century): Lavoisier was one of the first to carry out quantitatively accurate chemical measurements. He demonstrated that combustion required oxygen, and he demonstrated oxygen's role in the rusting of metals. His observations led him to deduce the following general law known as the law of conservation of mass:
2. law of definite proportions (Joseph Proust, shortly after Lavoisier): Proust studied metal compounds, including metal oxides, carbonates and sulfides. From the work of Robert Boyle in the 17th century, it was understood that substances that could be broken down into more fundamental components were mixtures or compounds. Substances that could not be further broken down were referred to as elements. Thus, Proust deduced the so-called law of definite proportions:
3. The law of multiple proportions: (John Dalton, shortly after Proust): Studied gases and gaseous mixtures under different external conditions. Building on Proust's work, he noted that mathematically discrete manner in which elements combined to form different compounds. For example, in carbon monoxide \((CO)\), the mass ratio of oxygen to carbon \(m_O/m_C =1.33\) and in carbon dioxide \((CO_2)\) \(m_O /m_C =2.66\). Thus, since the amount of carbon is fixed in each compound, we can look at how the amount of oxygen varies, and we find that \(m_O (in \ CO_2)/m_O(in \ CO)=2\). The generalization of this idea is the law of multiple proportions:
In every chemical transformation, an equal quantity of matter exists before and after the reaction.
(Because he was a tax collector and nobleman, Lavoisier was branded a traitor during the French Revolution and beheaded in 1794.)
In a given chemical compound, the proportion by mass of the elements that compose it are fixed, independent of the origin of the compound or its mode of preparation.This is basically saying that sodium chloride, for example, is always NaCl, no matter how it is obtained, made, or prepared. There are no ``intermediate'' compounds.
When two elements form a series of compounds, the masses of one that combine with a fixed mass of the other are in the ratio of (small) integers to each other.This law is obeyed by all gaseous compounds, which is what Dalton studied. Certain solids are exceptions to both this rule and the law of definite proportions. An example is the solid wüstite, which can range from \(Fe_{0.95} O\) to \(Fe_{0.85}O\), depending on the mode of preparation. These formulae express the incommensurate compositions possible in this solid. But, since atoms are essentially indestructible (we have to work hard to get them to fission!), this explains why we do not find compounds such as \(C_{13/7} H_{5/3}\) in nature. Dalton's observations led him to propose the notion of the atom as the fundamental and indestructible building blocks of matter.- 4. The law of combining volumes (Joseph Gay-Lussac, Amedeo Avagadro, Stanislao Cannizzaro, early 19th century following Dalton): Again, based on studies of gases and how they combine, the law of combining volumes has been attribued to these three. The generalization of the observations is stated as follows:
When two gases are allowed to react, such that the gases are at the same temperature and pressure, the volumes of each gas consumed will be in the ratio of small integers. Moreover, the ratio of the volume of each product gas to the volume of either reacting gas will be a ratio of simple integers.- Example: \(3 \ volumes \ of \ hydrogen \ + \ 1 \ volume \ of \ nitrogen \rightarrow 2 \ volumes \ of \ ammonia\).Although this is a statement about gases, its implications can be deduced: the coefficients in chemical equations expressions how much of different reactants combine to give products will be integers.
- 5. Avogadro's hypothesis (1811):
- Equal volumes of different gases (at the same temperature and pressure) contain equal numbers of particles.
Example: How many particles are in \(1 \ L \ of \ O_2\) gas, and how many particles are in \(1 \ L \ of \ H_2\) gas? The density of \(O_2 \ is \ 1.429 \ g/L\), and the density of \(H_2 \ gas \ is \ 0.0899 \ g/L\).
Solution: - \(mass \ of \ O_2 =(1 \ L)(1.429 \ g/L)=1.429 \ g/L\)
\(moles \ of \ O_2 =\dfrac{1.429 \ g}{31.998 \ g/mol}=0.04466 \ mol\)
\(molecules \ of \ O_2 =(0.04466 \ mol)(6.022 * 10^{23} \ molecules/mol)=2.69*10^{22} \ molecules\)
\(mass \ of \ H_2 =(1 \ L)(0.0899 \ g/L)=0.0899 \ g\)
\(moles \ of \ H_2 =\dfrac{0.0899 \ g}{2.0158 \ g/mol}=0.04466 \ mol\)
\(molecules \ of \ H_2 =(0.04466 \ mol)(6.022*10^{23} \ molecules/mol)=2.69*10^{22} \ molecules\)
Combining Avogadro's hypothesis with the law of combining volumes allows the statement written above about volumes of hydrogen and nitrogen combining to give ammonia as a statement about definite numbers of molecules rather then volumes. This number could be expressed as moles, or even as individual molecules. The statement then reads:
\[3H_2 +N_2 \rightarrow 2NH_3\]
This is an example of a balanced chemical equation. What does it mean to be balanced? It means that the law of conservation of mass is obeyed - equal quantities of hydrogen and nitrogen exist on both sides of the reaction. Furthermore, the coefficients, i.e., the numbers multiplying \(H_2\), \(N_2\) and \(NH_3\) in the above equation, are expressible as simple integers. When balancing chemical equations, this serves as an important check.Methods of balancing chemical equations
- 1. Balancing by inspection:Consider the example:
\[NH_4 NO_3 \rightarrow N_2 O +H_2 O\]
We notice that there are 2 nitrogens on both the left and right sides, so nitrogen is already balanced. There are 3 oxygens on the left but only 2 on the right, so oxygen is not balanced. Similarly, there are 4 hydrogens on the left and only 2 on the right, so hydrogen is not balanced. The balancing of oxygen and hydrogen can be handled together by making the coefficient of \(H_2 O\) equal to 2. Then, the balanced equation reads
\[NH_4 NO_3 \rightarrow N_2 O+2H_2 O\]
Now there are equal quantities of all elements on both sides of the reaction, and mass conservation is satisfied. Balancing by inspection in this way is quick and useful when you can see the solution easily.
2. Algebraic method:Consider the reaction:\[C_4 H_{10}+O_2 \rightarrow CO_2 +H_2 O\]
This equation could be balanced by inspection. However, we will use it to illustrate another approach - the algebraic approach. To balance a reaction algebraically, we start by putting unknown coefficients in front of each molecular species in the equation:
\[xC_4 H_{10}+yO_2 \rightarrow zCO_2 +wH_2 O\]
Then we write down the balance conditions for each element in terms of the unknowns. In this case, there are are four unknowns, \(x\), \(y\), \(z\) and \(w\). From carbon balance, we have the condition:
\[4x=z\]
The hydrogen balance condition is:
\[10x =2w\]
and the oxygen balance condition gives:
\[2y=2z+w\]
This constitutes a set of only three equations for the four unknowns. The fourth condition comes from recognizing that chemical equations specify relative amounts of reactants and products. Thus, one of the coefficients is arbitrary, and all the others can be expressed relative to it. Thus, we may take one of the coefficients to be 1, arbitrarily, which then leaves only three unknowns and three conditions to determine them. The choice of which coefficient to set to 1 is completely arbitrary and may be taken to be the one that is most convenient for solving the problem. In this case, the choice \(x=1\) allows us to arrive at a solution almost immediately, for then it follows that \(z=4\) and \(w=5\). From the oxygen balance condition, \(y\) can be determined to be \(2y=8+5=13\) so \(y=13/2\). Thus, the balanced equation could be written as
\[C_4 H_{10}+\dfrac{13}{2}O_2 \rightarrow 4CO_2 +5H_2 O\]
This is a perfectly fine way of writing the equation, however, customarily, it is preferable to express all the coefficients as integers, in accordance with the law of combining volumes and Avogadro's hypothesis. Thus, we can multiply through the equation by 2 on both sides (it can be treated as an algebraic equation in this way) and obtain the final result:
\[2C_4 H_{10}+13O_2 \rightarrow 8CO_2 +10H_2 O\]
As a second example, consider the reaction:
\[NaCl +SO_2 +H_2 O +O_2 \rightarrow Na_2 SO_4 +HCl\]
Again, we put unknown coefficients in front of each molecular species:
\[xNaCl +ySO_2 +zH_2 O+wO_2\rightarrow uNa_2 SO_4 +vHCl\]
Writing down the balance conditions on each element gives:Sodium balance:
\[x=2u\]
Chlorine balance:
\[x=v\]
Sulfur balance:
\[y=u\] Oxygen balance:
\[2y+z+2w=4u\] Hydrogen balance:
\[2z=v\]Setting \(u=1\) arbitrarily, gives the immediate solution:
\[x=2 \;\;\;\; v=2\;\;\;\; y=1\;\;\;\; z=1\] and \(2+1+2w=4\) or \(w=1/2\). In order to clear the fraction, we multiply all the coefficients by 2 and write down the balanced equation: \[4NaCl +2SO_2 +2H_2 O +O_2 \rightarrow 2Na_2 SO_4 +4HCl\]
Law of Conservation of Energy
The law of conservation of energy is one of the basic laws of physics and therefore governs the microscopic motion of individual atoms in a chemical reaction. The Law of Conservation Energy states:
Law of Conservation Energy
In a closed system, i.e., a system that isolated from its surroundings, the total energy of the system is conserved.In SI units, energy has units of Joules. \(1 \ Joule = 1 kg\cdot m^2 \cdot s^{-2}\).
Some forms of energy:
- Kinetic energy - energy of motion.
- Potential energy - energy of ``location'' with respect to some reference point.
- Chemical energy - energy stored in chemical bonds, which can be released in reactions.
- Electrical energy - energy created by separating charges; energy stored in a battery, for example.
- Thermal energy - energy given off as heat, such as friction.
Since everything has a microscopic origin, the last three are really special cases of potential and kinetic energies, however, the classification is useful.
The kinetic energy of an object of mass \(m\), moving with a velocity \(v\) is given by
\[KE=\dfrac{1}{2}m|v|^2\]
Recall that \(|v|\) is the speed \(v\) of the particle, so that the kinetic energy can be written as \(1/2 \ mv^2\).
Potential energy is a little less straightforward. Since it is an energy of location with respect to some reference point, the potential energy of an object depends on the specific situation. An example is gravity. An object of mass \(m\) at a height \(h\) above the Earth's surface has a gravitational potential energy
\[PE=mgh\]
where \(g\) is the acceleration due to the Earth's gravity. In this example, the potential energy has a simple form, namely that it depends linearly on the height \(h\), which describes the object's location and may even be varying in time if, for example, the object is actually falling toward the Earth.
In general, the potential energy may not be such a simple function of location. In this case, one needs a potential energy curve to describe the potential energy as a function of some coordinate describing the object's location. Consider the example of a mass attached to a spring moving in one spatial dimension. Let \(x\) represent the mass's position along the x-axis, and let \(x_0\) represent its equilibrium position. As the mass stretches the spring \((x>x_0)\), its potential energy increases, and as it compresses the spring compresses \((x<x_0)\), its potential energy increases as well. The potential energy can be described by a potential energy function \(V(x)\) that is symmetric about \(x=x_0\), as represented in the figure below:
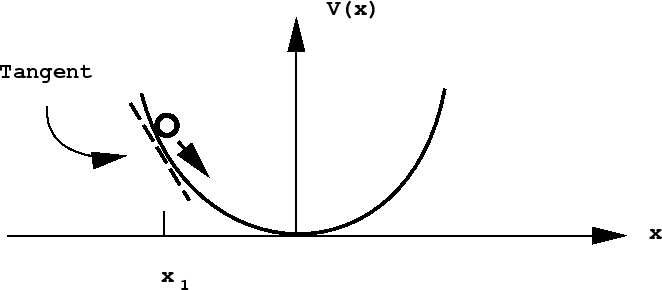
Notice, also, that the mass actually moves under the action of a force, which also changes as a function of \(x\). In fact, the force exerted by the spring on the mass can be determined from the potential energy curve via
\[Force \ at \ x=-slope \ of \ the \ potential \ energy \ curve \ at \ x\]
That is, the force is the slope of the line tangent to the curve at the point \(x\), as shown in the figure above for the point \(x_1\). In terms of a derivative, the force is given by
\[F=-\dfrac{dV}{dx}\]
Note that, at the bottom of the gully, where the curve is flat, the force is 0. The SI units of force are Newtons. \(1 \ N = 1 \ kg\cdot m \cdot s^{-2}\).
Consider the case of a diatomic molecule:
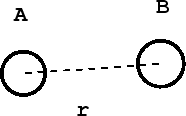
- Figure 2:
The distance between the atoms A and B is \(r\). The chemical forces that hold the molecule together come from a potential energy curve \(V(r)\) that depends only on the distance between them. Such a curve might look like:
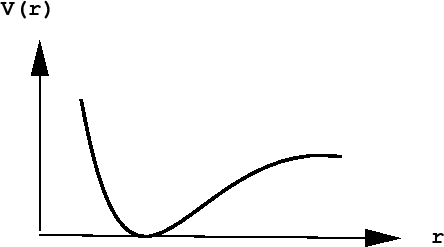
The presence of a deep ``well'' indicates a particular distance of lowest potential energy. This corresponds to the bond length of the molecule and is, therefore, the most likely value of the separation of the two atoms (think of a ball placed in a gully of this shape - if placed at the bottom of the well, the force on it would be 0, hence it would not move, but would remain there forever unless disturbed).
According to Newton's second law of motion, force is the action that causes a change in the motion of a particle or sets it in motion from rest. Consider a particle of mass \(m\) moving in one dimension so that its position is \(x\). If a force \(F\) acts on the particle, it will be set in motion, so that \(x\) is no longer a fixed number but a function \(x(t)\) of time \(t\). At any instant in time, the particle will have a velocity \(v\) defined to be the rate of change of \(x\) with respect to time
\[v(t)=\dfrac{dx}{dt}\]
In general \(v\) is not fixed but is also a function \(v(t)\) of time \(t\). If \(v(t)\) is not constant, in fact, then the particle also has an acceleration \(a\) defined as the rate of change of \(v\) with respect to time
\[a(t)=\dfrac{dv}{dt}\]
Since \(v=dx/dt\), it follows that
\[a(t)=\dfrac{d^2 x}{dt^2}\]
Newton's second law of motion states that the force \(F\) acting on the particle is directly proportion to this acceleration, with the mass \(m\) as the constant of proportionality
\[F=ma=m\dfrac{dv}{dt}=m\dfrac{d^2 x}{dt^2}\]
Since motion generally occurs in three rather than one dimension, position, velocity, acceleration, and force are all vector quantities. The position is usually denoted \(r\), and \(r\) has three components \(r=(x,y,z)\). Similarly velocity \(v\) has components \(v=(v_x ,v_y ,v_z)\), accleration \(a=(a_x ,a_y ,a_z)\), and \(F=(F_x ,F_y ,F_z)\). In vector notation, Newton's second law reads
\[F=ma=m\dfrac{dv}{dt}=m\dfrac{d^2 r}{dt^2}\]
Hence, if \(r\) is not a fixed vector but a function \(r(t)\) of time, this means that each component of \(r\) is a function of time \(r(t)=(x(t),y(t),z(t))\).
Work
Consider again a particle of mass \(m\) moving in one spatial dimension. If a force \(F\) is needed to move the particle from position \(x_1\) to position \(x_2\), then mechanical work \(W\) has been done on the particle. Since, as we have seen, \(F\) can be a function \(F(x)\) of \(x\), the general definition of work is the area under the force function \(F(x)\) between \(x_1\) and \(x_2\), i.e. the integral
\[W=\int_{x_1}^{x_2}F(x)dx\]
However, since \(F(x)=-dV/dx\), this becomes
\[W=-\int_{x_1}^{x_2}\dfrac{dV}{dx}dx=-[V(x_2)-V(x_1)]=V(x_1)-V(x_2)\]
Now if the velocity of the particle at \(x_1\) is \(v_1\) and at \(x_2\), it is \(v_2\), then by energy conservation
\[\dfrac{1}{2}mv_{1}^{2}+V(x_1)=\dfrac{1}{2}mv_{2}^{2}+V(x_2)\]
Rearranging this gives
\[V(x_1)-V(x_2)=\dfrac{1}{2}mv_{2}^{2}-\dfrac{1}{2}mv_{1}^{2}\]
Hence, the work \(W\) is also equal to the change in kinetic energy
\[W=\dfrac{1}{2}mv_{2}^{2}-\dfrac{1}{2}mv_{1}^{2}\]
a result known as the work-kinetic energy theorem.


