3.1: Isomers
- Page ID
- 288545
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Learning Outcomes
- Define isomer.
- Determine the isomeric relationship between a pair of molecules.
- Identify the chiral centers in a molecule.
- Describe different types of isomers.
One of the interesting aspects of organic chemistry is that it is three-dimensional. A molecule can have a shape in space that may contribute to its properties. Molecules can differ in the way the atoms are arranged - the same combination of atoms can be assembled in more than one way. These compounds are known as isomers. Isomers are molecules with the same molecular formulas, but different arrangements of atoms. There are several different types of isomers which will be described and a flowchart (see figure below) can help you determine which type of isomers are present.

Structural Isomers
A structural isomer, also known as a constitutional isomer, is one in which two or more organic compounds have the same molecular formulas but different structures. The two molecules below have the same chemical formula, but are different molecules because they differ in the location of the methyl group.
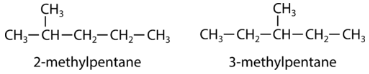
Alkenes can also demonstrate structural isomerism. In alkenes, there are multiple structural isomers based on where in the chain the double bond occurs. The condensed structural formulas of 1-butene and 2-butene show this.

The number in the name of the alkene refers to the lowest numbered carbon in the chain that is part of the double bond.
Stereoisomers
Stereoisomers have the same connectivity in their atoms but a different arrangement in three-dimensional space. There are different classifications of stereoisomers depending on how the arrangements differ from one another. Notice that in the structural isomers, there was some difference in the connection of atoms. For example, 1-butene has a double bond followed by two single bonds while 2-butene has a single bond, then a double bond, then a single bond. A stereoisomer will have the same connectivity among all atoms in the molecule.
Geometric Isomers
With a molecule such as 2-butene, a different type of isomerism called geometric isomerism can be observed. Geometric isomers are isomers in which the order of atom bonding is the same but the arrangement of atoms in space is different. The double bond in an alkene is not free to rotate because of the nature of the bond. Therefore, there are two different ways to construct the 2-butene molecule (see figure below). The image below shows the two geometric isomers, called cis-2-butene and trans-2-butene.
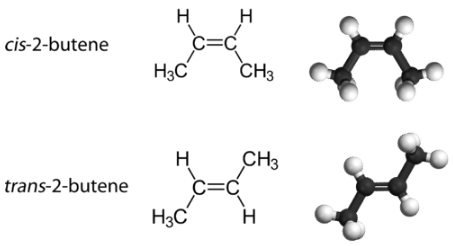
The cis isomer has the two single hydrogen atoms on the same side of the molecule, while the trans isomer has them on opposite sides of the molecule. In both molecules, the bonding order of the atoms is the same. In order for geometric isomers to exist, there must be a rigid structure in the molecule to prevent free rotation around a bond. This occurs with a double bond or a ring. In addition, the two carbon atoms must each have two different groups attached in order for there to be geometric isomers. Propene (see figure below) has no geometric isomers because one of the carbon atoms (the one on the far left) involved in the double bond has two single hydrogens bonded to it.
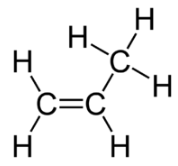
Physical and chemical properties of geometric isomers are generally different. As with alkenes, alkynes display structural isomerism beginning with 1-butyne and 2-butyne. However, there are no geometric isomers with alkynes because there is only one other group bonded to the carbon atoms that are involved in the triple bond.
Optical Isomers
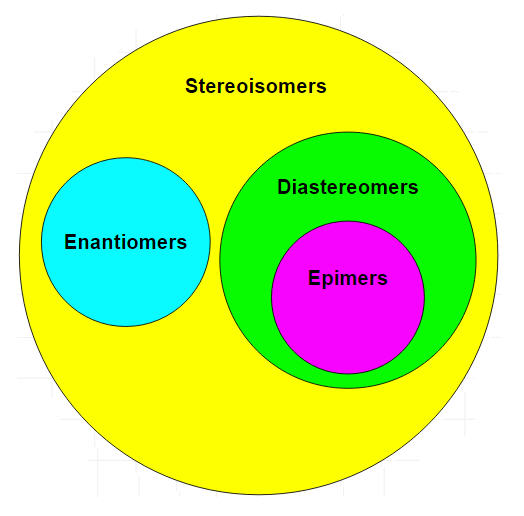
Stereoisomers that are not geometric isomers are known as optical isomers. Optical isomers differ in the placement of substituted groups around one or more atoms of the molecule. They were given their name because of their interactions with plane-polarized light. Optical isomers are labeled enantiomers or diastereomers.
Enantiomers are non-superimposable mirror images. A common example of a pair of enantiomers is your hands. Your hands are mirror images of one another but no matter how you turn, twist, or rotate your hands, they are not superimposable.
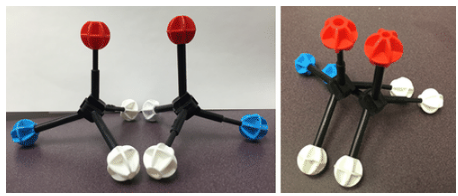
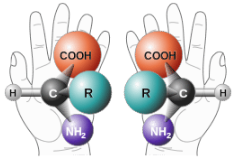
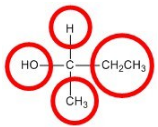
Objects that have non-superimposable mirror images are called chiral. When examining a molecule, carbon atoms with four unique groups attached are considered chiral. Look at the figure below to see an example of a chiral molecule. Note that we have to look beyond the first atom attached to the central carbon atom. The four circles indicate the four unique groups attached to the central carbon atom, which is chiral.
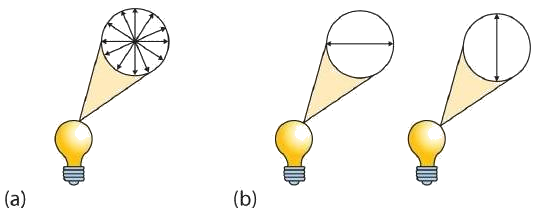
A beam of ordinary light can be pictured as a bundle of waves; some move up and down, some sideways, and others at all other conceivable angles. When a beam of light has been polarized, however, the waves in the bundle all vibrate in a single plane. Light altered in this way is called plane-polarized light. Much of what chemists know about stereoisomers comes from studying the effects they have on plane-polarized light. In this illustration, the light on the left is not polarized, while that on the right is polarized.
Sunlight, in general, is not polarized; light from an ordinary light bulb or an ordinary flashlight is not polarized. One way to polarize ordinary light is to pass it through Polaroid sheets, special plastic sheets containing carefully oriented organic compounds that permit only light vibrating in a single plane to pass through. To the eye, polarized light doesn’t “look” any different from nonpolarized light. We can detect polarized light, however, by using a second sheet of polarizing material, as shown here.

In the photo on the left, two Polaroid sheets are aligned in the same direction; plane-polarized light from the first Polaroid sheet can pass through the second sheet. In the photo on the right, the top Polaroid sheet has been rotated 90° and now blocks the plane-polarized light that comes through the first Polaroid sheet.
Certain substances act on polarized light by rotating the plane of vibration. Such substances are said to be optically active. The extent of optical activity is measured by a polarimeter, an instrument that contains two polarizing lenses separated by a sample tube, as shown in the accompanying figure. With the sample tube empty, maximum light reaches the observer’s eye when the two lenses are aligned so that both pass light vibrating in the same plane. When an optically active substance is placed in the sample tube, that substance rotates the plane of polarization of the light passing through it, so that the polarized light emerging from the sample tube is vibrating in a different direction than when it entered the tube. To see the maximum amount of light when the sample is in place, the observer must rotate one lens to accommodate the change in the plane of polarization.

Some optically active substances rotate the plane of polarized light to the right (clockwise) from the observer’s point of view. These compounds are said to be dextrorotatory; substances that rotate light to the left (counterclockwise) are levorotatory. To denote the direction of rotation, a positive sign (+) is given to dextrorotatory substances, and a negative sign (−) is given to levorotatory substances.
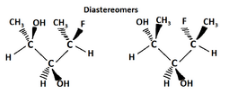
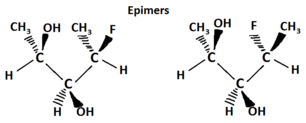
Another type of optical isomer are diastereomers, which are non-mirror image optical isomers. Diastereomers have a different arrangement around one or more atoms while some of the atoms have the same arrangement. As shown in the figure below, note that the orientation of groups on the first and third carbons are different but the second one remains the same so they are not the same molecule. The solid wedge indicates a group coming out of the page/screen towards you and the dashed line indicates that a group is going away from you "behind" the page/screen.
Epimers are a sub-group of diastereomers that differ at only one location. All epimers are diastereomers but not all diastereomers are epimers.
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)


