7.1: Carbohydrates
- Page ID
- 288509
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Recognizing & Classifying Carbohydrates
Carbohydrates contain only three elements: carbon, hydrogen, and oxygen. They also contain a limited number of functional groups. Each carbohydrate contains several alcohol groups and at least one aldehyde or ketone group. Despite the similarities between carbohydrate molecules, there are still many different carbohydrates due to the wide variety of sizes among carbohydrates. Because some carbohydrates are polymers these biomolecules can be classified as monosaccharides, disaccharides, and polysaccharides.
In addition to being the building blocks of larger carbohydrates, monosaccharides are often found in fruits, cane sugar, and honey. Examples of these molecules, also called simple sugars, include fructose and glucose. Monosaccharides can be classified by the number of carbon atoms (e.g. 3 = tri, 4 = tetra, etc.) and whether they contain an aldehyde or ketone functional group (an aldo- or keto- prefix is used). As you will see in the table below and throughout the carbohydrate section, names of these molecules often end in -ose.
|
Number of Carbon Atoms |
Aldose |
Ketose |
|---|---|---|
|
3 |
aldotriose |
ketotriose |
|
4 |
aldotetrose |
ketotetrose |
|
5 |
aldopentose |
ketopentose |
|
6 |
aldohexose |
ketohexose |
|
7 |
aldoheptose |
ketoheptose |
The images shown are called Fischer projections. They are similar to the skeletal structures introduced in the Organic Chemistry chapter, but also provide information about chirality. At each intersection of the lines there is a carbon atom; all other atoms are shown explicitly. The L- and D- forms of glucose are two different isomers (enantiomers).
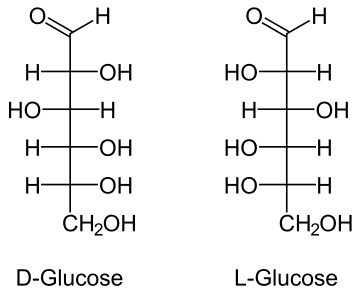
Each monosaccharide has several chiral carbon atoms. (Can you identify them in the examples above? Remember that a chiral center is a carbon atom with four different groups attached.) To distinguish between different forms prefixes L- and D- are used, for example L-glucose and D-glucose. Interestingly, most monosaccharides found in nature are the D- form. Monosaccharides often exist in a cyclic form rather than the straight chain forms. The cyclic form of D-glucose is shown below.

The ring is formed by a reaction that forms a single bond between the oxygen on carbon 5 and carbon 1 (where the aldehyde group was). All of the same atoms are present, but the hydrogen atom that had been part of the hydroxyl (-OH) group on carbon 5 is now bonded to the oxygen atom on carbon 1 (formally the carbonyl oxygen).
Disaccharides
As the name implies, disaccharides are carbohydrates made from two monosaccharides. A common example is lactose which contains the monomers glucose and galactose.

Polysaccharides
Polysaccharides are natural polymers consisting of many monosaccharide units. Examples include starch, cellulose, and glycogen. Starch is a complex carbohydrate found in potatoes and other foods. Cellulose gives structure to cell walls in plants and cannot be digested by humans because we cannot break the bond between the monosaccharide units. Glycogen is a polysaccharide produced in the body to store excess glucose for later use.
Amylose, a starch, is a polymer made from glucose monomers. Notice that the figure below has a glucose monomer in brackets with a subscipt of 300-600 indicating that there are hundreds of glocose monomers in a chain of amylose.
![Amylose, a polysaccharide consisting of hundreds of glucose units (designated by [glucose]_300-600)](https://chem.libretexts.org/@api/deki/files/408609/Screen_Shot_2022-06-06_at_11.58.07_AM.png?revision=1&size=bestfit&width=583&height=230)

