1.3: Classification of Matter
- Page ID
- 283307
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Learning Objectives
- Describe the solid, liquid and gas phases.
- Explain the difference between a pure substance and a mixture.
- Explain the difference between an element and a compound.
- Explain the difference between a homogeneous mixture and a heterogeneous mixture.
The States of Matter
Matter typically exists in one of three states: solid, liquid, or gas. The state a given substance exhibits is also a physical property. Some substances exist as gases at room temperature (oxygen and carbon dioxide), while others, like water and mercury metal, exist as liquids. Most metals exist as solids at room temperature. All substances can exist in any of these three states.



Note
Technically speaking a fourth state of matter called plasma exists, but it does not naturally occur on earth, so we will omit it from our study here.
Solid
Solids are defined by the following characteristics:
- Definite shape (rigid)
- Definite volume
- Particles vibrate around fixed axes
If we were to cool liquid mercury to its freezing point of \(-39^\text{o} \text{C}\), and under the right pressure conditions, we would notice all of the liquid particles would go into the solid state. Mercury can be solidified when its temperature is brought to its freezing point. However, when returned to room temperature conditions, mercury does not exist in solid state for long, and returns back to its more common liquid form.
Liquid
Liquids have the following characteristics:
- No definite shape (takes the shape of its container)
- Has definite volume
- Particles are free to move over each other, but are still attracted to each other
Mercury
A familiar liquid is mercury metal. Mercury is an anomaly. It is the only metal we know of that is liquid at room temperature. Mercury also has an ability to stick to itself (surface tension) - a property all liquids exhibit. Mercury has a relatively high surface tension, which makes it very unique. Here you see mercury in its common liquid form.
Video \(\PageIndex{1}\): Mercury boiling to become a gas.
If we heat liquid mercury to its boiling point of \(357^\text{o} \text{C}\), and under the right pressure conditions, we would notice all particles in the liquid state go into the gas state.
Gas
Gases have the following characteristics:
- No definite shape (takes the shape of its container)
- No definite volume
- Particles move in random motion with little or no attraction to each other
- Highly compressible
The characteristics of the three states of matter are listed in Table \(\PageIndex{1}\) and an animation of the movement and position of the individual particles is shown in Figure \(\PageIndex{2}\)
| Characteristics | Solids | Liquids | Gases |
|---|---|---|---|
| shape | definite | indefinite | indefinite |
| volume | definite | definite | indefinite |
| relative intermolecular interaction strength | strong | moderate | weak |
| relative particle positions | in contact and fixed in place | in contact but not fixed | not in contact, random positions |
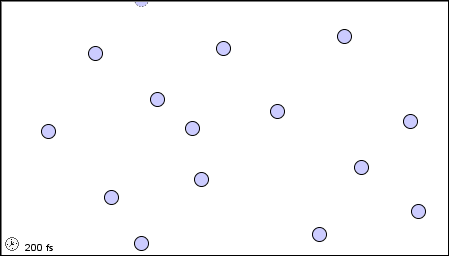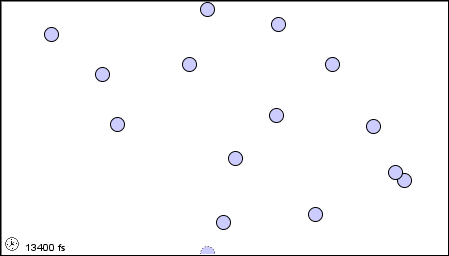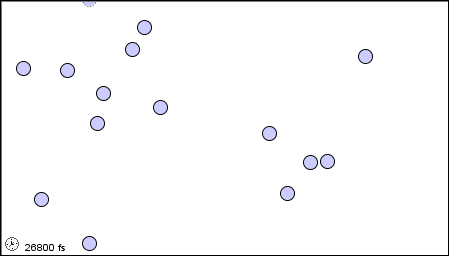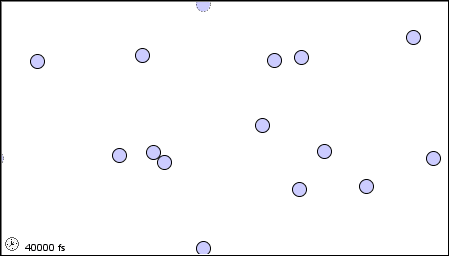 |
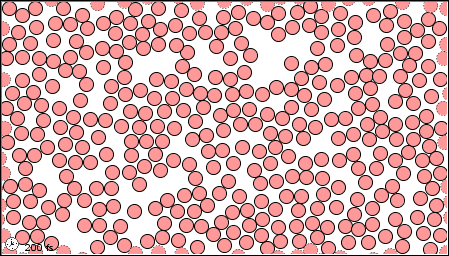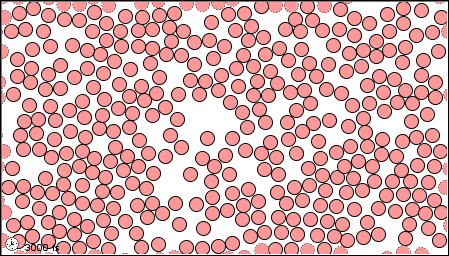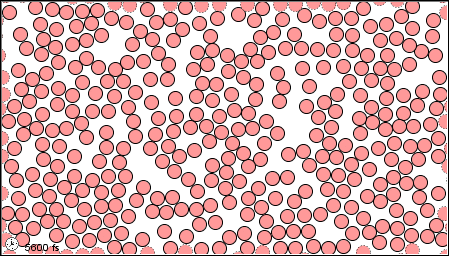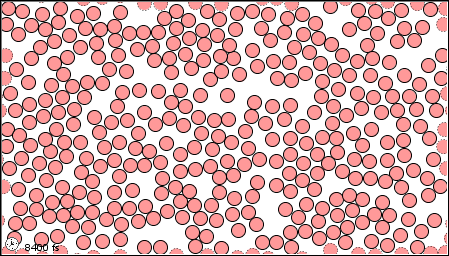 |
 |
| The gaseous state | The liquid state | The solid state |
Figure \(\PageIndex{2}\) A microscopic model showing particles (atoms or molecules) in the gaseous, liquid, and solid states.
Example \(\PageIndex{1}\)
What state or states of matter does each statement, describe?
- This state has a definite volume, but no definite shape.
- This state has no definite volume.
- This state allows the individual particles to move about while remaining in contact.
Solution
- This statement describes the liquid state.
- This statement describes the gas state.
- This statement describes the liquid state.
Exercise \(\PageIndex{1}\)
What state or states of matter does each statement describe?
- This state has individual particles in a fixed position with regard to each other.
- This state has individual particles far apart from each other in space.
- This state has a definite shape.
- Answer a:
- solid
- Answer b:
- gas
- Answer c:
- solid
Substances and Mixtures
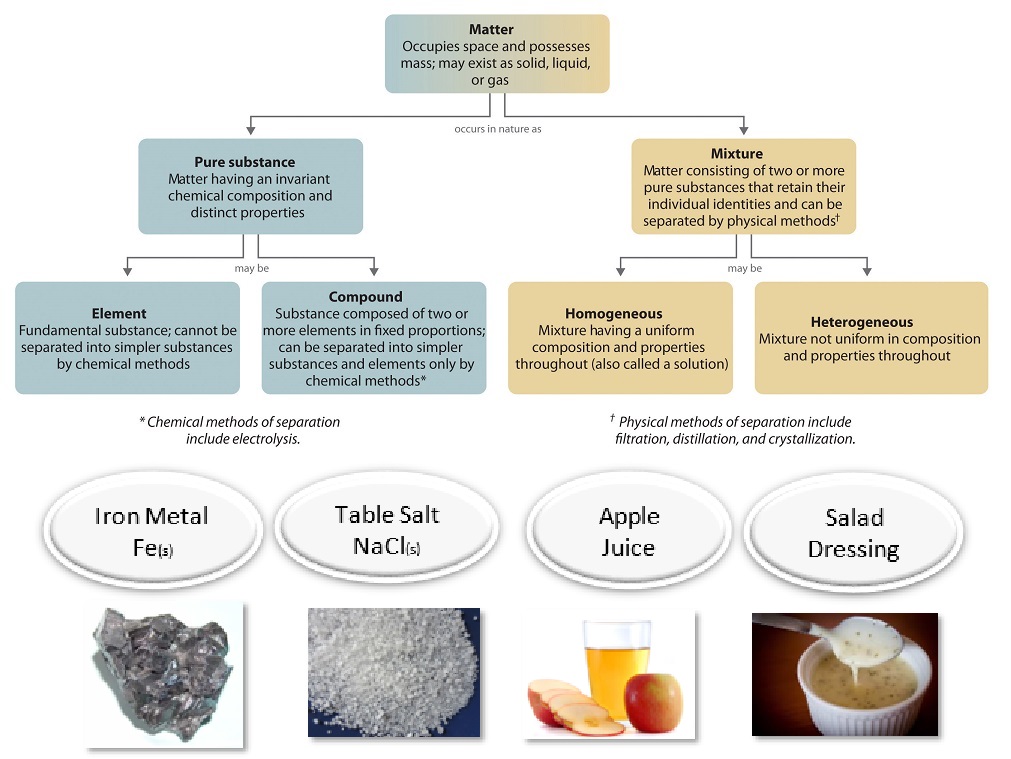
One useful way of organizing our understanding of matter is to think of a hierarchy that extends down from the most general and complex to the simplest and most fundamental (Figure \(\PageIndex{1}\)). Matter can be classified into two broad categories: pure substances and mixtures. A pure substance is a form of matter that has a constant composition (meaning it's the same everywhere) and properties that are constant throughout the sample (meaning there is only one set of properties such as melting point, color, boiling point, etc. throughout the matter). A material composed of two or more substances is a mixture.
Ordinary table salt is called sodium chloride. It is considered a pure substance because it has a uniform and definite composition. All samples of sodium chloride are chemically identical. Water is also a pure substance. Salt easily dissolves in water, but salt water cannot be classified as a pure substance because its composition can vary. You may dissolve a small amount of salt or a large amount into a given amount of water. A mixture is a physical blend of two or more components, each of which retains its own identity and properties in the mixture. Only the form of the salt is changed when it is dissolved into water. It retains its composition and properties.
A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. The difference is that the composition of the substance is always the same. The amount of salt in the salt water can vary from one sample to another. All solutions would be considered homogeneous because the dissolved material is present in the same amount throughout the solution.
A heterogeneous mixture is a mixture in which the composition is not uniform throughout the mixture. Vegetable soup is a heterogeneous mixture. Any given spoonful of soup will contain varying amounts of the different vegetables and other components of the soup.
Elements and Compounds
Elements and compounds are both examples of pure substances. A pure substance that cannot be broken down into chemically simpler components is an element. Aluminum, which is used in soda cans, is an element. A pure substance that can be broken down into chemically simpler components (because it has more than one element) is a compound. For example, water is a compound composed of the elements hydrogen and oxygen. Today, there are about 118 elements in the known universe. In contrast, scientists have identified tens of millions of different compounds to date.
Atoms, Elements, and Compounds
Video \(\PageIndex{2}\) Atoms, elements, and compounds (first five minutes of video)
Example \(\PageIndex{2}\)
Identify each substance as a compound, an element, a heterogeneous mixture, or a homogeneous mixture (solution).
- filtered tea
- freshly squeezed orange juice
- a compact disc
- aluminum oxide, a white powder that contains a 2:3 ratio of aluminum and oxygen atoms
- selenium
Given: a chemical substance
Asked for: its classification
Strategy:
- Decide whether a substance is chemically pure. If it is pure, the substance is either an element or a compound. If a substance can be separated into its elements, it is a compound.
- If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. If its composition is uniform throughout, it is a homogeneous mixture.
Solution:
- A) Tea is a solution of compounds in water, so it is not chemically pure. It is usually separated from tea leaves by filtration.
B) Because the composition of the solution is uniform throughout, it is a homogeneous mixture. - A) Orange juice contains particles of solid (pulp) as well as liquid; it is not chemically pure.
B) Because its composition is not uniform throughout, orange juice is a heterogeneous mixture. - A) A compact disc is a solid material that contains more than one element, with regions of different compositions visible along its edge. Hence a compact disc is not chemically pure.
B) The regions of different composition indicate that a compact disc is a heterogeneous mixture. - A) Aluminum oxide is a single, chemically pure compound.
- A) Selenium is one of the known elements.
Exercise \(\PageIndex{2}\)
Identify each substance as a compound, an element, a heterogeneous mixture, or a homogeneous mixture (solution).
- white wine
- mercury
- ranch-style salad dressing
- table sugar (sucrose)
- Answer a:
- homogeneous mixture (solution)
- Answer b:
- element
- Answer c:
- heterogeneous mixture
- Answer d:
- compound
Example \(\PageIndex{3}\)
How would a chemist categorize each example of matter?
- saltwater
- soil
- water
- oxygen
Solution
- Saltwater acts as if it were a single substance even though it contains two substances—salt and water. Saltwater is a homogeneous mixture, or a solution.
- Soil is composed of small pieces of a variety of materials, so it is a heterogeneous mixture.
- Water is a pure substance; more specifically, because water is composed of hydrogen and oxygen, it is a compound.
- Oxygen, a pure substance, is an element.
Exercise \(\PageIndex{3}\)
How would a chemist categorize each example of matter?
- coffee
- hydrogen
- an egg
- Answer a:
- a homogeneous mixture (solution), assume it's filtered coffee
- Answer b:
- element
- Answer c:
- heterogeneous mixture.
Summary
- Three states of matter exist - solid, liquid, and gas.
- Solids have a definite shape and volume.
- Liquids have a definite volume, but take the shape of the container.
- Gases have no definite shape or volume
-
Matter can be classified into two broad categories: pure substances and mixtures.
-
A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample.
-
Mixtures are physical combinations of two or more elements and/or compounds.
-
Mixtures can be classified as homogeneous or heterogeneous.
-
Elements and compounds are both examples of pure substances. Compounds are substances that are made up of more than one type of atom. Elements are the simplest substances made up of only one type of atom.
Contributors and Attributions
Stephen Lower, Professor Emeritus (Simon Fraser U.) Chem1 Virtual Textbook
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.
Henry Agnew (UC Davis)

