3.E: Electronic Structure and Periodic Properties (Exercises)
- Page ID
- 216742
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)These are homework exercises to accompany the Textmap created for "Chemistry" by OpenStax. Complementary General Chemistry question banks can be found for other Textmaps and can be accessed here. In addition to these publicly available questions, access to private problems bank for use in exams and homework is available to faculty only on an individual basis; please contact Delmar Larsen for an account with access permission.
6.1: Electromagnetic Energy
Q6.1.1
The light produced by a red neon sign is due to the emission of light by excited neon atoms. Qualitatively describe the spectrum produced by passing light from a neon lamp through a prism.
S6.1.1
The spectrum consists of colored lines, at least one of which (probably the brightest) is red.
S6.1.2
1.) Convert 614.5 nm into meters
\(6.145 nm\) = \(6.145 \times10^{-7} m\)
6.2: The Bohr Model
Q6.2.1
Why is the electron in a Bohr hydrogen atom bound less tightly when it has a quantum number of 3 than when it has a quantum number of 1?
Q6.2.2
What does it mean to say that the energy of the electrons in an atom is quantized?
S6.2.2
Quantized energy means that the electrons can possess only certain discrete energy values; values between those quantized values are not permitted.
Q6.2.3
The spectra of hydrogen and of calcium are shown in[link]. What causes the lines in these spectra? Why are the colors of the lines different? Suggest a reason for the observation that the spectrum of calcium is more complicated than the spectrum of hydrogen
S6.2.3
Both involve a relatively heavy nucleus with electrons moving around it, although strictly speaking, the Bohr model works only for one-electron atoms or ions. According to classical mechanics, the Rutherford model predicts a miniature “solar system” with electrons moving about the nucleus in circular or elliptical orbits that are confined to planes. If the requirements of classical electromagnetic theory that electrons in such orbits would emit electromagnetic radiation are ignored, such atoms would be stable, having constant energy and angular momentum, but would not emit any visible light (contrary to observation). If classical electromagnetic theory is applied, then the Rutherford atom would emit electromagnetic radiation of continually increasing frequency (contrary to the observed discrete spectra), thereby losing energy until the atom collapsed in an absurdly short time (contrary to the observed long-term stability of atoms). The Bohr model retains the classical mechanics view of circular orbits confined to planes having constant energy and angular momentum, but restricts these to quantized values dependent on a single quantum number, n. The orbiting electron in Bohr’s model is assumed not to emit any electromagnetic radiation while moving about the nucleus in its stationary orbits, but the atom can emit or absorb electromagnetic radiation when the electron changes from one orbit to another. Because of the quantized orbits, such “quantum jumps” will produce discrete spectra, in agreement with observations.
6.3: Development of Quantum Theory
Q6.3.1
Answer the following questions:
- Without using quantum numbers, describe the differences between the shells, subshells, and orbitals of an atom.
- How do the quantum numbers of the shells, subshells, and orbitals of an atom differ?
Q6.3.2
Identify the subshell in which electrons with the following quantum numbers are found:
- n = 2, l = 1
- n = 4, l = 2
- n = 6, l = 0
S6.3.2
(a) 2p; (b) 4d; (c) 6s
6.4: Electronic Structure of Atoms (Electron Configurations)
Q6.4.1
Read the labels of several commercial products and identify monatomic ions of at least four transition elements contained in the products. Write the complete electron configurations of these cations.
Q6.4.2
Read the labels of several commercial products and identify monatomic ions of at least six main group elements contained in the products. Write the complete electron configurations of these cations and anions.
S6.4.2
For example, Na+: 1s22s22p6; Ca2+: 1s22s22p6; Sn2+: 1s22s22p63s23p63d104s24p64d105s2; F–: 1s22s22p6; O2–: 1s22s22p6; Cl–: 1s22s22p63s23p6.
Q6.4.3
Using complete subshell notation (not abbreviations, 1s22s22p6, and so forth), predict the electron configuration of each of the following atoms:
- C
- P
S6.4.3
a.) 1s22s22p2
b.) 1s22s22p63s23p3
Q6.4.4
Using complete subshell notation (1s22s22p6, and so forth), predict the electron configuration of each of the following atoms:
- N
- Si
S6.4.4
- 1s22s22p3;
- 1s22s22p63s23p2;
Q6.4.5
Is 1s22s22p6 the symbol for a macroscopic property or a microscopic property of an element? Explain your answer.
Q6.4.6
What additional information do we need to answer the question “Which ion has the electron configuration 1s22s22p63s23p6”?
S6.4.6
The charge on the ion.
Q6.4.7
Draw the orbital diagram for the valence shell of each of the following atoms:
- C
- P
Q6.4.8
Use an orbital diagram to describe the electron configuration of the valence shell of each of the following atoms:
- N
- Si
S6.4.8
(a)
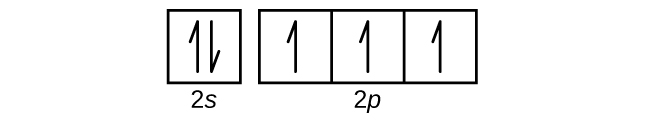
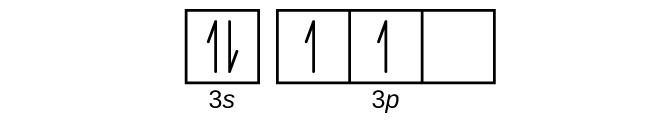
Q6.4.9
Using complete subshell notation (1s22s22p6, and so forth), predict the electron configurations of the following ions.
- N3–
- Ca2+
- S–
Q6.4.10
Which atom has the electron configuration: 1s22s22p63s23p64s23d104p65s24d2? Hint - how many electrons are in this atom?
S6.4.10
Zr
Q6.4.11
Which atom has the electron configuration: 1s22s22p63s23p63d74s2?
S6.4.11
Co ; Cobalt
Q6.4.12
Which ion with a +1 charge has the electron configuration 1s22s22p63s23p63d104s24p6? Which ion with a –2 charge has this configuration?
S6.4.12
Rb+, Se2−
Q6.4.13
Which of the following atoms contains only three valence electrons: Li, B, N, F, Ne?
S6.4.13
B ; Boron
Q6.4.14
Which of the following has two unpaired electrons?
- Mg
- Si
- S
- Both Mg and S
- Both Si and S.
S6.4.14
Although both (b) and (c) are correct, (e) encompasses both and is the best answer.
Q6.4.15
Which atom would be expected to have a half-filled 6p subshell?
S6.4.15
Bi ; Bismuth
Q6.4.16
Which atom would be expected to have a half-filled 4s subshell?
S6.4.16
K
Q6.4.17
In one area of Australia, the cattle did not thrive despite the presence of suitable forage. An investigation showed the cause to be the absence of sufficient cobalt in the soil. Cobalt forms cations in two oxidation states, Co2+ and Co3+. How many electrons are in each of these ions? How many protons?
Q6.4.18
Thallium was used as a poison in the Agatha Christie mystery story “The Pale Horse.” Thallium has two possible cationic forms, +1 and +3. The +1 compounds are the more stable. How many electrons are in each of these ions? How many protons?
Q6.4.19
Write the electron configurations for the following atoms or ions:
- B3+
- O–
- Cl3-
Q6.4.20
Cobalt–60 and iodine–131 are radioactive isotopes commonly used in nuclear medicine. How many protons, neutrons, and electrons are in atoms of these isotopes?
S6.4.20
Co has 27 protons, 27 electrons, and 33 neutrons.
I has 53 protons, 53 electrons, and 78 neutrons.
Based on their positions in the periodic table, predict which has the smallest atomic radius: Mg, Sr, Si, Cl, I.
S6.5.1
Cl
Q6.5.2
Based on their positions in the periodic table, predict which has the largest atomic radius: Li, Rb, N, F, I.
Q6.5.3
Based on their positions in the periodic table, predict which has the largest first ionization energy: Mg, Ba, B, O, Te.
S6.5.3
O
Q6.5.4
Based on their positions in the periodic table, predict which has the smallest first ionization energy: Li, Cs, N, F, I.
Q6.5.5
Based on their positions in the periodic table, rank the following atoms in order of increasing first ionization energy: F, Li, N, Rb
S6.5.5
Rb < Li < N < F
Q6.5.6
Based on their positions in the periodic table, rank the following atoms or compounds in order of increasing first ionization energy: Mg, O, S, Si
Q6.5.7
Atoms of which group in the periodic table have a valence shell electron configuration of ns2np3?
S6.5.7
5A
Q6.5.8
Atoms of which group in the periodic table have a valence shell electron configuration of ns2?
Q6.5.9
Based on their positions in the periodic table, list the following atoms in order of increasing radius: Mg, Ca, Rb, Cs.
S6.5.9
Mg < Ca < Rb < Cs
Q6.5.10
Based on their positions in the periodic table, list the following atoms in order of increasing radius: Sr, Ca, Si, Cl.
Q6.5.11
Based on their positions in the periodic table, list the following ions in order of increasing radius: K+, Ca2+, Al3+, Si4+.
S6.5.11
Si4+ < Al3+ < Ca2+ < K+
Q6.5.12
List the following ions in order of increasing radius: Li+, Mg2+, Br–, Te2–.
Q6.5.15
Compare both the numbers of protons and electrons present in each to rank the following ions in order of increasing radius: As3–, Br–, K+, Mg2+.
S6.5.15
Mg2+ < K+ < Br– < As3–
Q6.5.18
The ionic radii of the ions S2–, Cl–, and K+ are 184, 181, 138 pm respectively. Explain why these ions have different sizes even though they contain the same number of electrons.


