7.5: Photochemical Smog- Making Haze While the Sun Shines
- Last updated
- Save as PDF
- Page ID
- 344025
Learning Objectives
- Describe photochemical smog.
- List different means to address photochemical smog.
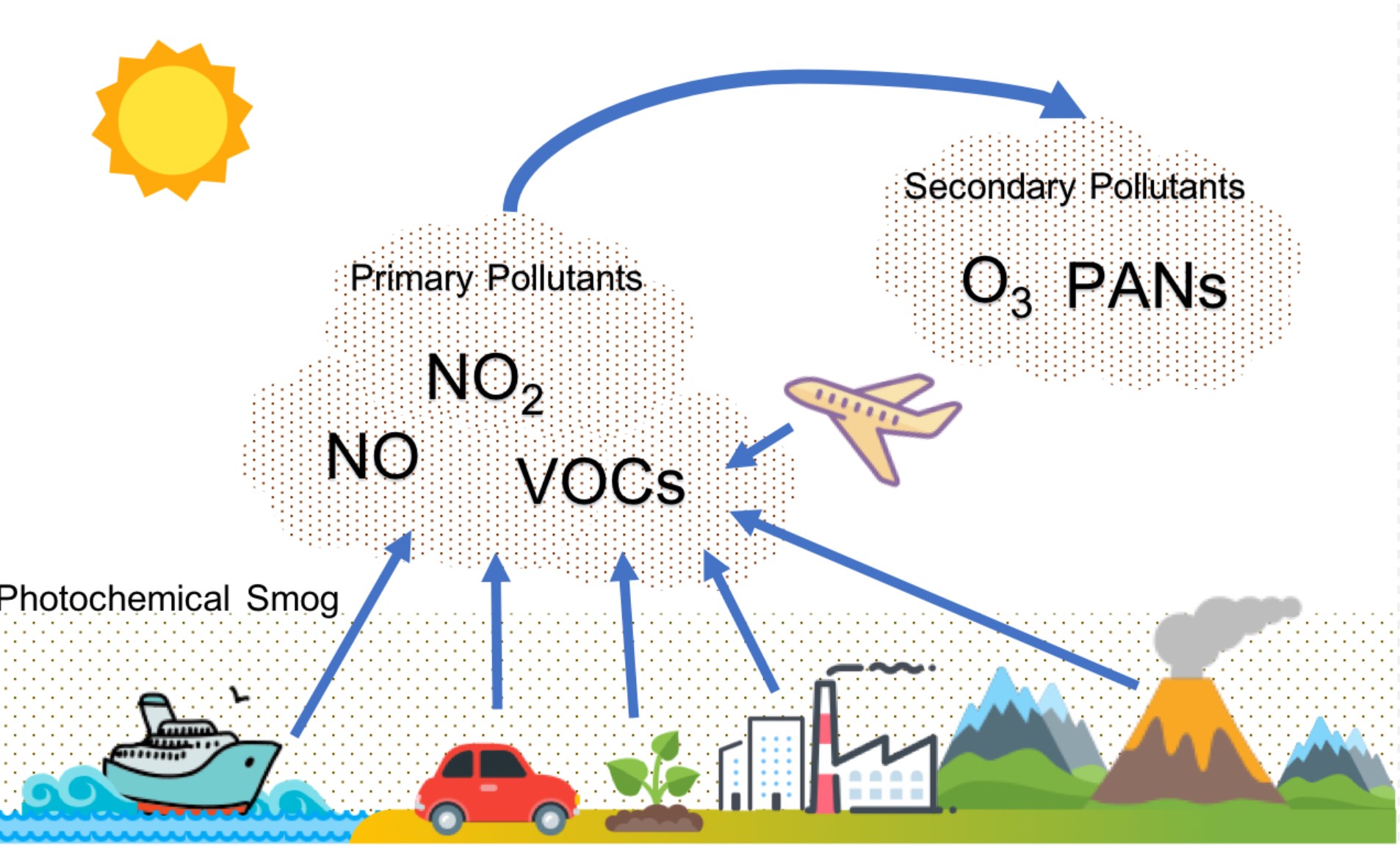
Photochemical smog is a type of air pollution due to the reaction of solar radiation with airborne pollutant mixtures of nitrogen oxides (NOx) and volatile organic compounds (hydrocarbons). Smog is a byproduct of modern industrialization. Due to industry and the number of motor vehicles, this is more of a problem in large cities that have a warm, sunny and dry climate.
- Oxidation: Photochemical smog is also referred to as oxidizing smog. Oxidation reactions have been defined several ways. In terms of oxygen transfer, oxidation is a gain of oxygen. Oxidation can also be defined as a loss of hydrogen. The most important use of oxidation is described in terms of electron transfer. Oxidation can be described as an increase in oxidation number or loss of electrons. Oxidation numbers represents a distribution of charge. In other words, oxidation numbers represent the charge of the atom if the compound was composed of ions.
- Reduction: Reduction can involve the gain of hydrogen or loss of oxygen. Reduction can refer to the gain of electrons, which results in a decrease in oxidation number.
Formation of Photochemical Smog
The different reactions involved in the formation of photochemical smog are given below.
Step 1: People begin driving in the morning, nitrogen is burned or oxidized
\[N_2 + O_2 \rightarrow 2NO \nonumber \]
- Oxidation number of N2 is 0. The nitrogen in NO has acquired an oxidation number of +2.
Step 2: After a few hours, NO combines with O2, in another oxidation reaction
\[2NO + O_2 \rightarrow 2NO_2 \nonumber \]
- The nitrogen in NO has an oxidation number of +2. The nitrogen in NO2 has an oxidation number of +4.
Step 3: Nitrogen dioxide absorbs light energy, resulting in a reduction reaction
\[NO_2 \rightarrow NO + O \nonumber \]
- The nitrogen in NO2 has an oxidation number of +4 and the nitrogen in NO is +2.
Step 4: In sunlight, atomic oxygen combines with oxygen gas to form ozone
\[O + O_2 \rightarrow O_3 \nonumber \]
Step 5: Reaction is temperature and sunlight dependent
\[O_3 + NO \rightleftharpoons NO_2 + O_2 \nonumber \]
Alternative Reactions
NO and NO2 can also react with the hydrocarbons instead of ozone to form other volatile compounds known as PAN (peroxyacetyl nitrate) as shown in Figure . The accumulation of ozone and volatile organic compounds along with the energy from the sun forms the brown, photochemical smog seen on hot, sunny days. Panoramic view of Santiago covered by a layer of smog on May 10, 2006. The Metropolitan Region of Santiago facing the driest autumn last 28 years due to lack of rainfall, which coupled with poor air circulation, causes an increase in smog.
Health Hazards
Because ozone is highly reactive, it has the ability to oxidize and destroy lung tissue. Short term exposures to elevated levels of ozone (above .75 ppm) have been linked to a host of respiratory irritations including coughing, wheezing, substernal soreness, pharyngitis, and dyspnea. Prolonged exposure to the molecule has been proven to cause a permanent reduction in lung function, as well as elevate the risk of developing asthma. Sulfur dioxide is a common component of London smog. Epidemiological studies have linked short term sulfur dioxide exposure to respiratory irritations including coughing, wheezing, and pharyngitis.
Other Harmful Effects of Smog
Plants are harmed by exposure to nitrogen oxides, ozone, and peroxyacetyl nitrate (PAN, see above), all oxidants present in a smoggy atmosphere. PAN is the most harmful of these constituents, damaging younger plant leaves, especially. Ozone exposure causes formation of yellow spots on leaves, a condition called chlorotic stippling. Some plant species, including sword-leaf lettuce, black nightshade, quickweed, and double-fortune tomato, are extremely susceptible to damage by oxidant species in smog and are used as bioindicators of the presence of smog. Costs of crop and orchard damage by smog run into millions of dollars per year in areas prone to this kind of air pollution, such as southern California.
Materials that are adversely affected by smog are generally those that are attacked by oxidants. The best example of such a material is rubber, especially natural rubber, which is attacked by ozone. Indeed, the hardening and cracking of natural rubber has been used as a test for atmospheric ozone.
Visibility-reducing atmospheric aerosol particles are the most common manifestation of the harm done to atmospheric quality by smog. The smog-forming process occurs by the oxidation of organic materials in the atmosphere, and carbon-containing organic materials are the most common constituents of the aerosol particles in an atmosphere afflicted by smog. Conifer trees(pine and cypress) and citrus trees are major contributors to the organic hydrocarbons that are precursors to organic particle formation in smog.
Controlling Photochemical Smog
Every new vehicle sold in the United States must include a catalytic converter to reduce photochemical emissions. Catalytic converters force CO and incompletely combusted hydrocarbons to react with a metal catalyst, typically platinum, to produce CO2 and H2O. Additionally, catalytic converters reduce nitrogen oxides from exhaust gases into O2 and N2, eliminating the cycle of ozone formation. Many scientists have suggested that pumping gas at night could reduce photochemical ozone formation by limiting the amount of exposure VOCs have with sunlight.
Preventing Smog with Green Chemistry
Smog is basically a chemical problem, which would indicate that it should be amenable to chemical solutions. Indeed, the practice of green chemistry and the application of the principles of industrial ecology can help to reduce smog. This is due in large part to the fact that a basic premise of green chemistry is to avoid the generation and release of chemical species with the potential to harm the environment. The best way to prevent smog formation is to avoid the release of nitrogen oxides and organic vapors that enable smog to form. At an even more fundamental level, measures can be taken to avoid the use of technologies likely to release such substances, for example, by using alternatives to polluting automobiles for transportation.
The evolution of automotive pollution control devices to reduce smog provides an example of how green chemistry can be used to reduce pollution. The first measures taken to reduce hydrocarbon and nitrogen oxide emissions from automobiles were very much command-and-control and “end-of-pipe” measures. These primitive measures implemented in the early 1970s did reduce emissions, but with a steep penalty in fuel consumption and in driving performance of vehicles. However, over the last three decades, the internal combustion automobile engine has evolved into a highly sophisticated computer-controlled machine that generally performs well, emits few air pollutants, and is highly efficient. (And it would be much more efficient if those drivers who feel that they must drive “sport utility” behemoths would switch to vehicles of a more sensible size.) This change has required an integrated approach involving reformulation of gasoline. The first major change was elimination from gasoline of tetraethyllead, an organometallic compound that poisoned automotive exhaust catalysts (and certainly was not good for people). Gasoline was also reformulated to eliminate excessively volatile hydrocarbons and unsaturated hydrocarbons (those with double bonds between carbon atoms) that are especially reactive in forming photochemical smog.
An even more drastic approach to eliminating smog-forming emissions is the use of electric automobiles that do not burn gasoline. These vehicles certainly do not pollute as they are being driven, but they suffer from the probably unsolvable problem of a very limited range between charges and the need for relatively heavy batteries. However, hybrid automobiles using a small gasoline or diesel engine that provides electricity to drive electric motors propelling the automobile and to recharge relatively smaller batteries can largely remedy the emission and fuel economy problems with automobiles. The internal combustion engine on these vehicles runs only as it is needed to provide power and, in so doing, can run at a relatively uniform speed that provides maximum economy with minimum emissions.
Another approach that is being used on vehicles as large as buses that have convenient and frequent access to refueling stations is the use of fuel cells that can generate electricity directly from the catalytic combination of elemental hydrogen and oxygen, producing only harmless water as a product . There are also catalytic process that can generate hydrogen from liquid fuels, such as methanol, so that vehicles carrying such a fuel can be powered by electricity generated in fuel cells.
Green chemistry can be applied to devices and processes other than automobiles to reduce smog-forming emissions. This is especially true in the area of organic solvents used for parts cleaning and other industrial operations, vapors of which are often released to the atmosphere. The substitution of water with proper additives or even the use of supercritical carbon dioxide fluid can eliminate such emissions.
Summary
- Photochemical smog is a mixture of pollutants that are formed (mostly during the hot summer months) when nitrogen oxides and volatile organic compounds (VOCs) react to sunlight, creating a brown haze above cities.
- Photochemical smog is formed from the reactions of natural and man-made emissions of nitrogen oxides and VOCs.
- Smog is a serious problem in many cities and continues to harm human health and are especially harmful for senior citizens, children, and people with heart and lung conditions such as emphysema, bronchitis, and asthma.
- Catalytic converters in gas powered vehicles help reduce photochemical emissions.
- The practice of green chemistry and the application of the principles of industrial ecology can help to reduce smog.
Contributors and Attributions
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.
- Stanley Manahan (University of Missouri)
- Wikipedia

