7.2: Chemistry of the Atmosphere
- Last updated
- Save as PDF
- Page ID
- 344022
Learning Objectives
- Describe the nitrogen cycle.
- Describe the oxygen cycle.
- Describe the conditions for temperature inversion.
The mix of gases in the atmosphere forms a complex system organized into layers that together support life on Earth. Although there are numerous gases, as shown in Table 13.1.1, the top four gases make up 99.998 % of the volume of clean dry air (unpolluted air that does not contain water vapor). Of this dry composition of the atmosphere nitrogen, by far, is the most common (78%). Nitrogen dilutes oxygen and prevents rapid or instantaneous burning at the Earth's surface, as oxygen gas is a necessary reactant of the combustion process. Nitrogen is also needed and used by living things to make proteins, though as nitrogen gas, N2, it is unavailable to most living things. Oxygen is used by all living things to make molecules that are essential for life. It is also essential for aerobic respiration as well as combustion or burning.
Nitrogen Cycle
All life requires nitrogen-compounds, e.g., proteins and nucleic acids. Air, which is 79% nitrogen gas (N2), is the major reservoir of nitrogen. But most organisms cannot use nitrogen in this form. Figure \(\PageIndex{1}\) illustrates the entire nitrogen cycle. Plants must secure their nitrogen in "fixed" form, i.e., incorporated in compounds such as: nitrate ions (NO3−), ammonium ions (NH4+) and urea (NH2)2CO. Animals secure their nitrogen (and all other) compounds from plants (or animals that have fed on plants).
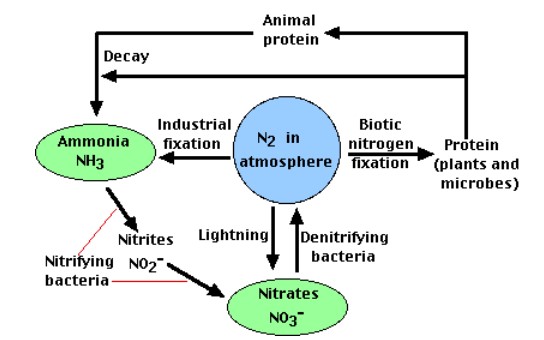
Four processes participate in the cycling of nitrogen through the biosphere: (1) nitrogen fixation, (2) decay, (3) nitrification, and (4) denitrification. Microorganisms play major roles in all four of these.
Nitrogen Fixation
The nitrogen molecule (N2) is quite inert. To break it apart so that its atoms can combine with other atoms requires the input of substantial amounts of energy. Three processes are responsible for most of the nitrogen fixation in the biosphere:
- atmospheric fixation by lightning
- biological fixation by certain microbes alone or in a symbiotic relationship with some plants and animals
- industrial fixation
Atmospheric Fixation
The enormous energy of lightning breaks nitrogen molecules and enables their atoms to combine with oxygen in the air forming nitrogen oxides. These dissolve in rain, forming nitrates, that are carried to the earth. Atmospheric nitrogen fixation probably contributes some 5– 8% of the total nitrogen fixed.
Industrial Fixation
Under great pressure, at a temperature of 600°C, and with the use of a catalyst, atmospheric nitrogen and hydrogen (usually derived from natural gas or petroleum) can be combined to form ammonia (NH3). Ammonia can be used directly as fertilizer, but most of its is further processed to urea and ammonium nitrate (NH4NO3).
Biological Fixation
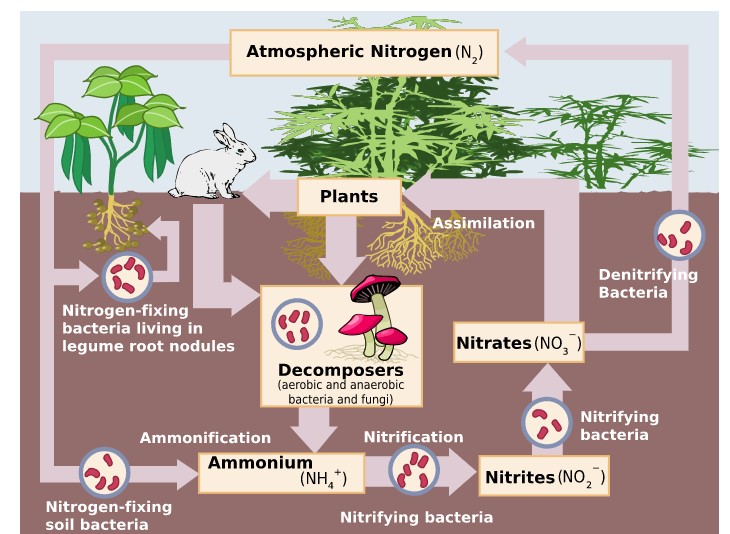
The ability to fix nitrogen in the soil (Figure \(\PageIndex{2}\) ) only in certain bacteria and archaea.
- Some live in a symbiotic relationship with plants of the legume family (e.g., soybeans, alfalfa).
- Some establish symbiotic relationships with plants other than legumes (e.g., alders).
- Some establish symbiotic relationships with animals, e.g., termites and "shipworms" (wood-eating bivalves).
- Some nitrogen-fixing bacteria live free in the soil.
- Nitrogen-fixing cyanobacteria are essential to maintaining the fertility of semi-aquatic environments like rice paddies.
Biological nitrogen fixation requires a complex set of enzymes and a huge expenditure of ATP. Although the first stable product of the process is ammonia, this is quickly incorporated into protein and other organic nitrogen compounds.
Decay
The proteins made by plants enter and pass through food webs just as carbohydrates do. At each trophic level, their metabolism produces organic nitrogen compounds that return to the environment, chiefly in excretions. The final beneficiaries of these materials are microorganisms of decay. They break down the molecules in excretions and dead organisms into ammonia.
Nitrification
Ammonia can be taken up directly by plants — usually through their roots. However, most of the ammonia produced by decay is converted into nitrates. Until recently this was thought always to be accomplished in two steps:
- Bacteria of the genus Nitrosomonas oxidize \(\ce{NH3}\) to nitrites (\(\ce{NO2^{−}}\)).
- Bacteria of the genus Nitrobacter oxidize the nitrites to nitrates (\(\ce{NO3^{−}}\)).
These two groups of autotrophic bacteria are called nitrifying bacteria. Through their activities (which supply them with all their energy needs), nitrogen is made available to the roots of plants. However, in 2015, two groups reported finding that bacteria in the genus Nitrospira were able to carry out both steps: ammonia to nitrite and nitrite to nitrate. This ability is called "comammox" (for complete ammonia oxidation).
In addition, both soil and the ocean contain archaeal microbes, assigned to the Crenarchaeota, that convert ammonia to nitrites. They are more abundant than the nitrifying bacteria and may turn out to play an important role in the nitrogen cycle.
Many legumes, in addition to fixing atmospheric nitrogen, also perform nitrification - converting some of their organic nitrogen to nitrites and nitrates. These reach the soil when they shed their leaves.
Denitrification
The three processes above remove nitrogen from the atmosphere and pass it through ecosystems. Denitrification reduces nitrates and nitrites to nitrogen gas, thus replenishing the atmosphere. In the process several intermediates are formed:
- nitric oxide (NO)
- nitrous oxide (N2O)(a greenhouse gas 300 times as potent as CO2)
- nitrous acid (HONO)
Once again, bacteria are the agents. They live deep in soil and in aquatic sediments where conditions are anaerobic. They use nitrates as an alternative to oxygen for the final electron acceptor in their respiration.
Anammox (anaerobic ammonia oxidation)
Under anaerobic conditions in marine and freshwater sediments, other species of bacteria are able to oxidize ammonia (with \(\ce{NO2^{−}}\)) forming nitrogen gas.
\[\ce{NH4^{+} + NO2^{−} → N2 + 2H2O} \nonumber \]
The anammox reaction may account for as much as 50% of the denitrification occurring in the oceans. All of these processes participate in closing the nitrogen cycle.
Are the denitrifiers keeping up?
Agriculture may now be responsible for one-half of the nitrogen fixation on earth through the use of fertilizers produced by industrial fixation and the the growing of legumes like soybeans and alfalfa. This is a remarkable influence on a natural cycle. Are the denitrifiers keeping up the nitrogen cycle in balance? Probably not. Certainly, there are examples of nitrogen enrichment in ecosystems. One troubling example: the "blooms" of algae in lakes and rivers as nitrogen fertilizers leach from the soil of adjacent farms (and lawns). The accumulation of dissolved nutrients in a body of water is called eutrophication.
Oxygen Cycle
Oxygen is the most abundant element on the earth’s crust. The earth’s surface is composed of the crust, atmosphere, and hydrosphere. About 50% of the mass of the earth’s crust consists of oxygen (combined with other elements, principally silicon). Oxygen occurs as O2 molecules and, to a limited extent, as O3 (ozone) molecules in air. It forms about 20% of the mass of the air. About 89% of water by mass consists of combined oxygen. In combination with carbon, hydrogen, and nitrogen, oxygen is a large part of plants and animals.
Oxygen is a colorless, odorless, and tasteless gas at ordinary temperatures. It is slightly denser than air. Although it is only slightly soluble in water (49 mL of gas dissolves in 1 L at STP), oxygen’s solubility is very important to aquatic life.
Oxygen is essential in combustion processes such as the burning of fuels. Plants and animals use the oxygen from the air in respiration (Figure \(\PageIndex{4}\)). The main way free oxygen is lost from the atmosphere is via and , mechanisms in which life and consume oxygen and release carbon dioxide.
The respiration process is represented as:
\[\ce{6O2+C6H12O6→6CO2+6H2O} \nonumber \]
Green plants continually replenish the oxygen in the atmosphere by a process called photosynthesis (Figure \(\PageIndex{3}\)) . The products of photosynthesis may vary, but, in general, the process converts carbon dioxide and water into glucose (a sugar) and oxygen using the energy of light:
&\ce{6CO2}(g) \:+\: &&\ce{6H2O}(l)
\:\mathrm{\underset{light}{\xrightarrow{chlorophyll}}}\:
&&\ce{C6H12O6}(aq) \:+\: &&\ce{6O2}(g)\\
&\mathrm{carbon\\ dioxide} &&\ce{water} &&\ce{glucose} &&\ce{oxygen}
\end{alignat} \nonumber \]

Overview of and photosynthesis (green) and respiration (red).
Water (at right), together with carbon dioxide (CO2), form oxygen and organic compounds (at left),
which can be respired to water and (CO2). Source: Wikipedia
Thus, the oxygen that became carbon dioxide and water by the metabolic processes in plants and animals returns to the atmosphere by photosynthesis. Photosynthesizing organisms include the plant life of the land areas as well as the of the oceans. The tiny marine was discovered in 1986 and accounts for more than half of the photosynthesis of the open ocean.
Oxygen is a key reactant in various oxidation reactions mentioned in section 8.5. Atmospheric free oxygen is also consumed by chemical weathering and surface reactions. An example of surface weathering is formation of rust:
Ozone forms naturally in the upper atmosphere by the action of ultraviolet light from the sun on the oxygen there. Most atmospheric ozone occurs in the stratosphere, a layer of the atmosphere extending from about 10 to 50 kilometers above the earth’s surface. This ozone acts as a barrier to harmful ultraviolet light from the sun by absorbing it via a chemical decomposition reaction:
\[\ce{O3}(g)\xrightarrow{\ce{ultraviolet\: light}}\ce{O}(g)+\ce{O2}(g) \nonumber \]
Temperature Inversions
In meteorology, an inversion, also known as a temperature inversion, is a deviation from the normal change of an amospheric property with altitude. It almost always refers to an inversion of the thermal lapse rate. Normally, air temperature decreases with an increase in altitude. During an inversion, warmer air is held above cooler air; the normal temperature profile with altitude is inverted.
An inversion traps air pollution, such as smog, close to the ground. An inversion can also suppress convection by acting as a "cap". If this cap is broken for any of several reasons, convection of any moisture present can then erupt into violent thunderstorms Temperature inversion can notoriously result in freezing rain in cold climates.
Summary
- The different forms of nitrogen that can be used in metabolism are produced through the process of nitrogen fixation.
- Bacteria in the soil carry out a process known as denitrification which converts nitrates back to nitrogen gas.
- Oxygen is produced mainly through photosynthesis. An additional source of atmospheric free oxygen comes from , whereby high-energy radiation breaks down atmospheric water and nitrous oxide.
- Oxygen is utilized during cellular respiration and decay. As well as during chemical weathering and various oxidation reactions.
- Temperature inversion is when a layer of cool air is trapped under a layer of warmer air. The cool dense air can trap and accumulate air pollutants.
Contributors and Attributions
- Template:ContribKimball
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- TextMap: Microbiology (Boundless)
- Wikipedia

