9.3.1: Melting, Freezing, and Sublimation
- Page ID
- 367834
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define melting, freezing, and sublimation.
A phase change is a physical process in which a substance goes from one phase to another. In this subsection, we will be focusing on the transitions between liquids and solids, as well as between solids and gases. The process of a solid becoming a liquid is called melting (an older term that you may see sometimes is fusion). The opposite process, a liquid becoming a solid, is called freezing (some textbooks also use the word solidification for this process). Although we associate the term freezing with low temperatures and melting with high temperatures, the temperature at which these changes occur depends upon the substance. For example, bacon grease becomes solid at room temperature; we could say that it freezes even though we do not experience that temperature as being “cold.” Even more extreme, many metals freeze at temperatures greater than 1000°C! (And based on what we have already covered related to crystalline solids, hopefully you can predict that metals will freeze at much higher temperatures than water.)
Usually the phase change occurs when adding or removing heat at a particular temperature, known as the melting point or the boiling point of the substance. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a liquid to a solid). The boiling point is the temperature at which a substance goes from a liquid to a gas (or from a gas to a liquid). The nature of the phase change depends on the direction of the heat transfer. Heat going into a substance changes it from a solid to a liquid, or a liquid to a gas. Removing heat from a substance changes a gas to a liquid, or a liquid to a solid.
Two key points are worth emphasizing. First, at a substance’s melting point or boiling point, two phases can exist simultaneously. Take water (H2O) as an example. On the Celsius scale, H2O has a melting point of 0°C and a boiling point of 100°C. At 0°C, both the solid and liquid phases of H2O can coexist. However, if heat is added, some of the solid H2O will melt and turn into liquid H2O. If heat is removed, the opposite happens: some of the liquid H2O turns into solid H2O. A similar process can occur at 100°C: adding heat increases the amount of gaseous H2O, while removing heat increases the amount of liquid H2O (Figure \(\PageIndex{1}\)).

Second, the temperature of a substance does not change as the substance goes from one phase to another. In other words, phase changes are isothermal (isothermal means “constant temperature”). Again, consider H2O as an example. Solid water (ice) can exist at 0°C. If heat is added to ice at 0°C, some of the solid changes phase to make liquid, which is also at 0°C. Remember, the solid and liquid phases of H2O can coexist at 0°C. Only after all of the solid has melted into liquid does the addition of heat change the temperature of the substance.
For each phase change of a substance, there is a characteristic quantity of heat needed to perform the phase change per gram (or per mole) of material. The heat of fusion (ΔHfus) is the amount of heat per gram (or per mole) required for a phase change that occurs at the melting point. Calculations related to the heat of fusion will be covered in a later subsection.
Melting Point
Solids are similar to liquids in that both are condensed states, with particles that are far closer together than those of a gas. However, while liquids are fluid, solids are not. The particles of most solids are packed tightly together in an orderly arrangement. The motion of individual atoms, ions, or molecules in a solid is restricted to vibrational motion about a fixed point. Solids are almost completely incompressible and are the most dense of the three states of matter.
As a solid is heated, its particles vibrate more rapidly as the solid absorbs kinetic energy. Eventually, the organization of the particles within the solid structure begins to break down and the solid starts to melt. The melting point is the temperature at which a solid changes into a liquid. At its melting point, the disruptive vibrations of the particles of the solid overcome the attractive forces operating within the solid. As with boiling points, the melting point of a solid is dependent on the strength of those attractive forces. Sodium chloride \(\left( \ce{NaCl} \right)\) is an ionic compound that consists of a multitude of strong ionic bonds. Sodium chloride melts at \(801^\text{o} \text{C}\). Ice (solid \(\ce{H_2O}\)) is a molecular compound composed of molecules that are held together by hydrogen bonds. Though hydrogen bonds are the strongest of the intermolecular forces, the strength of hydrogen bonds is much less than that of ionic bonds. The melting point of ice is \(0^\text{o} \text{C}\).
The melting point of a solid is the same as the freezing point of the liquid. At that temperature, the solid and liquid states of the substance are in equilibrium. For water, this equilibrium occurs at \(0^\text{o} \text{C}\).
\[\ce{H_2O} \left( s \right) \rightleftharpoons \ce{H_2O} \left( l \right)\]
We tend to think of solids as those materials that are solid at room temperature. However, all materials have melting points of some sort. Gases become solids at extremely low temperatures, and liquids will also become solid if the temperature is low enough. The table below gives the melting points of some common materials.
| Materials | Melting Point (ºC) |
|---|---|
| Hydrogen | -259 |
| Oxygen | -219 |
| Diethyl ether | -116 |
| Ethanol | -114 |
| Water | 0 |
| Pure silver | 961 |
| Pure gold | 1063 |
| Iron | 1538 |
- Explain what happens when heat flows into or out of a substance at its melting point or boiling point.
- How does the amount of heat required for a phase change relate to the mass of the substance?
- Answer a
-
The energy goes into changing the phase, not the temperature.
- Answer b
-
The amount of heat is a constant per gram of substance.
Sublimation
Under some circumstances, the solid phase can transition directly to the gas phase without going through a liquid phase, and a gas can directly become a solid. The solid-to-gas change is called sublimation, while the reverse process is called deposition. Sublimation is isothermal, like the other phase changes. There is a measurable energy change during sublimation—this energy change is called the enthalpy of sublimation, represented as ΔHsub. The relationship between the ΔHsub and the other enthalpy changes is as follows:
\[ΔH_{sub} = ΔH_{fus} + ΔH_{vap}\nonumber \]
As such, ΔHsub is not always tabulated because it can be simply calculated from ΔHfus and ΔHvap.
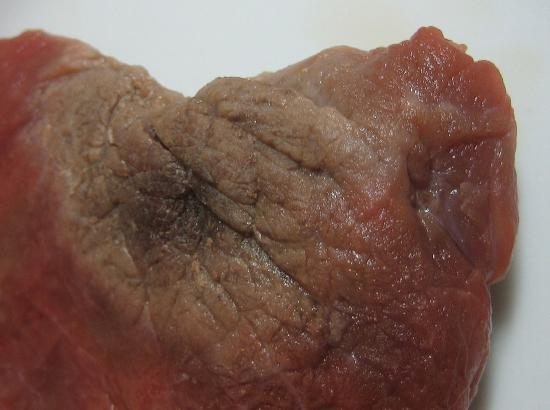
There are several common examples of sublimation. A well-known product, dry ice, is actually solid CO2. Dry ice is dry because it sublimes, with the solid bypassing the liquid phase and going straight to the gas phase. The sublimation occurs at temperature of −77°C, so it must be handled with caution. If you have ever noticed that ice cubes in a freezer tend to get smaller over time, it is because the solid water is very slowly subliming. "Freezer burn" isn't actually a burn; it occurs when certain foods, such as meats, slowly lose solid water content because of sublimation. The food is still good, but looks unappetizing. Reducing the temperature of a freezer will slow the sublimation of solid water.
Summary
- There is an energy change associated with any phase change.
- Sublimation is the change of state from a solid to a gas, without passing through the liquid state.
- Deposition is the change of state from a gas to a solid.
- Carbon dioxide is an example of a material that easily undergoes sublimation.
- The melting point is the temperature at which a solid changes into a liquid.
- Intermolecular forces have a strong influence on melting point.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:


