9.1: Chemical Equations
- Last updated
- Save as PDF
- Page ID
- 118824
Learning Objectives
- Identify the reactants and products in any chemical reaction.
- Convert word equations into chemical equations.
- Use the common symbols, \(\left( s \right)\), \(\left( l \right)\), \(\left( g \right)\), \(\left( aq \right)\), and \(\rightarrow\) appropriately when writing a chemical reaction.
In a chemical change, new substances are formed. In order for this to occur, the chemical bonds of the substances break, and the atoms that compose them separate and rearrange themselves into new substances with new chemical bonds. When this process occurs, we call it a chemical reaction. A chemical reaction is the process in which one or more substances are changed into one or more new substances.
Reactants and Products
To describe a chemical reaction, we need to indicate what substances are present at the beginning and what substances are present at the end. The substances that are present at the beginning are called reactants and the substances present at the end are called products.
Sometimes when reactants are put into a reaction vessel, a reaction will take place to produce products. Reactants are the starting materials, that is, whatever we have as our initial ingredients. The products are just that—what is produced—or the result of what happens to the reactants when we put them together in the reaction vessel. If we think about baking chocolate chip cookies, our reactants would be flour, butter, sugar, vanilla, baking soda, salt, egg, and chocolate chips. What would be the products? Cookies! The reaction vessel would be our mixing bowl.
\[ \underbrace{\text{Flour} + \text{Butter} + \text{Sugar} + \text{Vanilla} + \text{Baking Soda} + \text{Eggs} + \text{Chocolate Chips}}_{\text{Ingredients = Reactants}} \rightarrow \underbrace{\text{Cookies}}_{\text{Product}} \nonumber \]
Writing Chemical Equations
When sulfur dioxide is added to oxygen, sulfur trioxide is produced. Sulfur dioxide and oxygen, \(\ce{SO_2} + \ce{O_2}\), are reactants and sulfur trioxide, \(\ce{SO_3}\), is the product.
\[ \underbrace{\ce{2 SO2(g) + O2(g) }}_{\text{Reactants}} \rightarrow \underbrace{\ce{2SO3(g)}}_{\text{Products}} \nonumber \]
In chemical reactions, the reactants are found before the symbol "\(\rightarrow\)" and the products are found after the symbol "\(\rightarrow\)". The general equation for a reaction is:
\[\text{Reactants } \rightarrow \text{Products} \nonumber \]
There are a few special symbols that we need to know in order to "talk" in chemical shorthand. In the table below is the summary of the major symbols used in chemical equations. Table \(\PageIndex{1}\) shows a listing of symbols used in chemical equations.
| Symbol | Description | Symbol | Description |
|---|---|---|---|
| \(+\) | used to separate multiple reactants or products | \(\left( s \right)\) | reactant or product in the solid state |
| \(\rightarrow\) | yield sign; separates reactants from products | \(\left( l \right)\) | reactant or product in the liquid state |
| \(\rightleftharpoons\) | replaces the yield sign for reversible reactions that reach equilibrium | \(\left( g \right)\) | reactant or product in the gas state |
| \(\overset{\ce{Pt}}{\rightarrow}\) | formula written above the arrow is used as a catalyst in the reaction | \(\left( aq \right)\) | reactant or product in an aqueous solution (dissolved in water) |
| \(\overset{\Delta}{\rightarrow}\) | triangle indicates that the reaction is being heated |
Chemists have a choice of methods for describing a chemical reaction.
1. They could draw a picture of the chemical reaction.
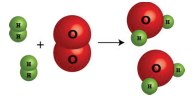
2. They could write a word equation for the chemical reaction:
"Two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of water vapor."
3. They could write the equation in chemical shorthand.
\[2 \ce{H_2} \left( g \right) + \ce{O_2} \left( g \right) \rightarrow 2 \ce{H_2O} \left( g \right) \nonumber \]
In the symbolic equation, chemical formulas are used instead of chemical names for reactants and products, while symbols are used to indicate the phase of each substance. It should be apparent that the chemical shorthand method is the quickest and clearest method for writing chemical equations.
We could write that an aqueous solution of calcium nitrate is added to an aqueous solution of sodium hydroxide to produce solid calcium hydroxide and an aqueous solution of sodium nitrate. Or in shorthand we could write:
\[\ce{Ca(NO_3)_2} \left( aq \right) + 2 \ce{NaOH} \left( aq \right) \rightarrow \ce{Ca(OH)_2} \left( s \right) + 2 \ce{NaNO_3} \left( aq \right) \nonumber \]
How much easier is that to read? Let's try it in reverse. Look at the following reaction in shorthand and write the word equation for the reaction:
\[\ce{Cu} \left( s \right) + \ce{AgNO_3} \left( aq \right) \rightarrow \ce{Cu(NO_3)_2} \left( aq \right) + \ce{Ag} \left( s \right) \nonumber \]
The word equation for this reaction might read something like "solid copper reacts with an aqueous solution of silver nitrate to produce a solution of copper (II) nitrate with solid silver."
To turn word equations into symbolic equations, we need to follow the given steps:
- Identify the reactants and products. This will help you know which symbols go on each side of the arrow and where the \(+\) signs go.
- Write the correct formulas for all compounds. You will need to use the rules you learned in Chapter 5 (including making all ionic compounds charge balanced).
- Write the correct formulas for all elements. Usually this is given straight off of the periodic table. However, there are seven elements that are considered diatomic, meaning that they are always found in pairs in nature. They include those elements listed in the table.
| Element Name | Hydrogen | Nitrogen | Oxygen | Fluorine | Chlorine | Bromine | Iodine |
|---|---|---|---|---|---|---|---|
| Formula | \(H_2\) | \(N_2\) | \(O_2\) | \(F_2\) | \(Cl_2\) | \(Br_2\) | \(I_2\) |
Example \(\PageIndex{1}\)
Transfer the following symbolic equations into word equations or word equations into symbolic equations.
- \(\ce{HCl} \left( aq \right) + \ce{NaOH} \left( aq \right) \rightarrow \ce{NaCl} \left( aq \right) + \ce{H_2O} \left( l \right)\)
- Gaseous propane, \(\ce{C_3H_8}\), burns in oxygen gas to produce gaseous carbon dioxide and liquid water.
- Hydrogen fluoride gas reacts with an aqueous solution of potassium carbonate to produce an aqueous solution of potassium fluoride, liquid water, and gaseous carbon dioxide.
Solution
a. An aqueous solution of hydrochloric acid reacts with an aqueous solution of sodium hydroxide to produce an aqueous solution of sodium chloride and liquid water.
b. Reactants: propane (\(\ce{C_3H_8}\)) and oxygen (\(\ce{O_2}\))
Product: carbon dioxide (\(\ce{CO_2}\)) and water (\(\ce{H_2O}\))
\[\ce{C_3H_8} \left( g \right) + \ce{O_2} \left( g \right) \rightarrow \ce{CO_2} \left( g \right) + \ce{H_2O} \left( l \right) \nonumber \]
c. Reactants: hydrogen fluoride and potassium carbonate
Products: potassium fluoride, water, and carbon dioxide
\[\ce{HF} \left( g \right) + \ce{K_2CO_3} \left( aq \right) \rightarrow \ce{KF} \left( aq \right) + \ce{H_2O} \left( l \right) + \ce{CO_2} \left( g \right) \nonumber \]
Exercise \(\PageIndex{1}\)
Transfer the following symbolic equations into word equations or word equations into symbolic equations.
- Hydrogen gas reacts with nitrogen gas to produce gaseous ammonia.
- \(\ce{HCl} \left( aq \right) + \ce{LiOH} \left( aq \right) \rightarrow \ce{LiCl} \left( aq \right) + \ce{H_2O} \left( l \right)\)
- Copper metal is heated with oxygen gas to produce solid copper(II) oxide.
- Answer a
- \(H_2 (g) + N_2 (g) \rightarrow NH_3 (g)\)
- Answer b
- An aqueous solution of hydrochloric acid reacts with an aqueous solution of lithium hydroxide to produce an aqueous solution of lithium chloride and liquid water.
- Answer c
- \(Cu (s) + O_2 (g) \rightarrow CuO (s)\)
Summary
- A chemical reaction is the process by which one or more substances are changed into one or more new substances.
- Chemical reactions are represented by chemical equations.
- Chemical equations have reactants on the left, an arrow that is read as "yields", and the products on the right.

